"The most expensive medicine in history"!Price of 19.08 million yuan
Author:Hebei Daily Time:2022.08.20
Recently, the US Food and Drug Administration (FDA) approved the Blue Bird Biological Corporation (Blue, a stock price of $ 5.81 and a market value of $ 448 million).
ZyNTEGLO's pricing in the United States is as high as US $ 2.8 million (about RMB 19.08 million), exceeding the Nuohua Pharmaceutical (NVS, a stock price of $ 84.92, and a market value of US $ 185.362 billion). The most expensive medicine.
This therapy is a disposable gene therapy, also known as "Beti-Cel", which is used to treat all genotype β-thalassene anemia that requires regular blood transfusion. This is the first genetic therapy for FDA to approve β-thalassemia patients. Bloomberg said that for such patients, the therapy "changed the rules of the game." Prior to this, the only available treatment for the disease was to rely on the patient's loved ones for bone marrow transplantation.
Bluebird creatures were born in the wave of gene therapy. Once, as the "darling" of the biotechnology community, Wall Street investors are rushing, but now they fall to the edge of funds.
Under the stimulus of ZyNTEGLO's news, the stock price of Blue Bird's biological stock rose 24%on August 17, local time, and once again rose 17%before the next day, but closed on the 18th, the stock price fell 14.31%, the acquisition was closed, and the acquisition was closed. 5.81 USD/share. As of the closing on the 19th, the stock price rebounded slightly, up 2.75%to close $ 5.97.
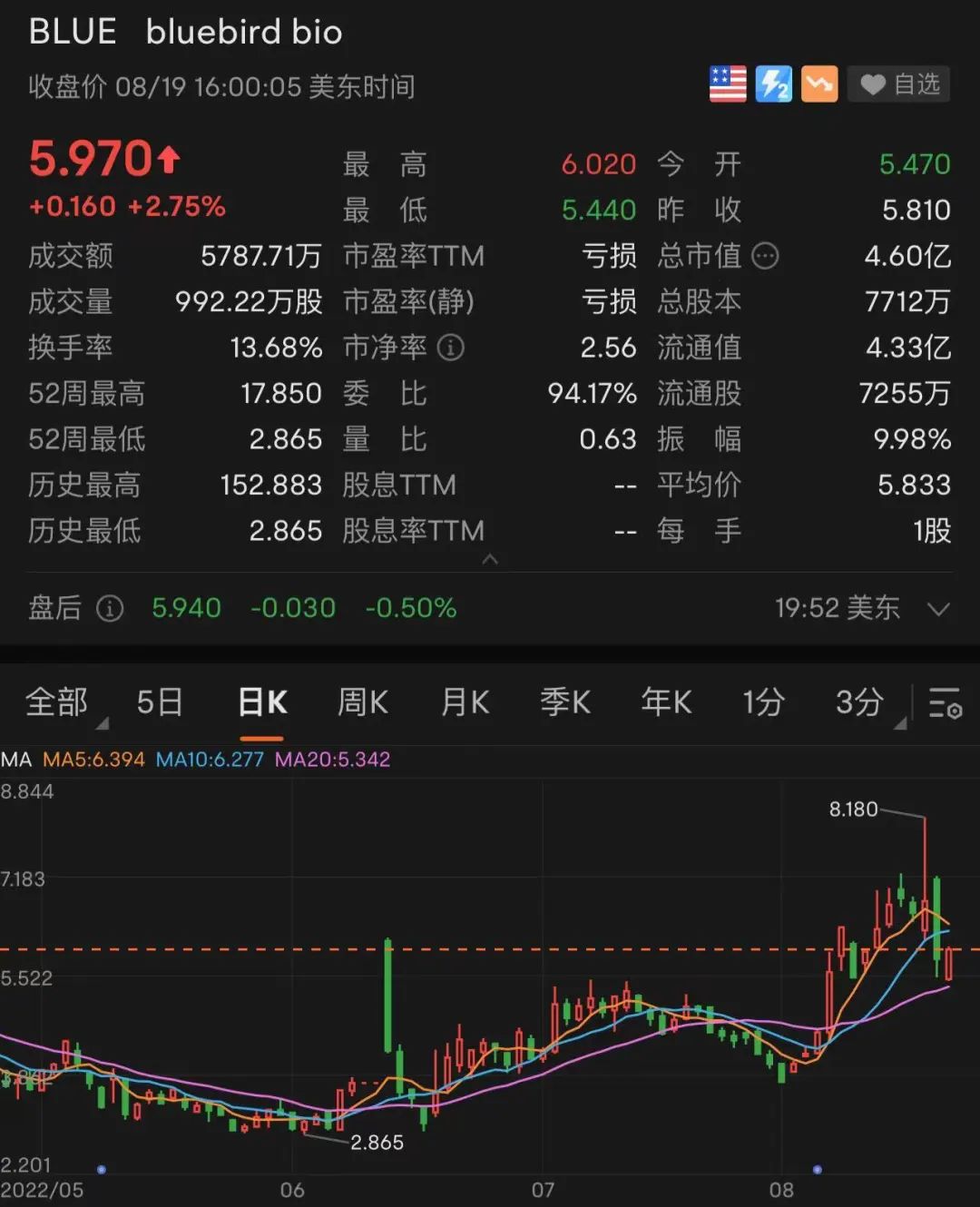
Today, can the blue bird creatures "take off" again with this "most expensive medicine in history"?

Photo source: Photo Network-400077451

"Change the rules of the game"
In June of this year, the FDA consultant group unanimously voted to support the approval of the listing of ZyNTEGLO, saying that the benefits of this treatment greater than risk. According to the FDA, 89%of patients receiving ZyNTEGLO gene therapy in the later trial can not have to be transfusion without any obvious safety issues.
Alexis Thompson, head of the Department of Hematology of Philadelphia Children's Hospital, said that although the gene therapy is not effective for everyone, the life of most patients has undergone tremendous changes. Philadelphia Children's Hospital used the therapy for treatment in clinical trials. Thompson revealed that before that, beta-patients with blood transfusion need to stay in the hospital for 4 to 6 hours in the hospital every 2 to 4 weeks, and sometimes they need to be hospitalized overnight.
According to reports, β-thalassemia is one of the most common single-gene diseases. Due to mutations in the genes encoding β-protein, the hemoglobin level in the patient's body decreased significantly or even missing. In order to survive, patients had to receive blood transfusion treatment for life. Although blood transfusion can temporarily relieve symptoms related to severe anemia, including fatigue, weakness, and shortness of breath, the cure is not cured, and severe complications caused by iron overload and multi -organ damage may occur.
Over the past ten years, the median death age of patients with blood transfusion dependencies in the United States is 37 years old.
ZYNTEGLO is a disposable gene therapy. The β-pearl protein gene is imported into the patient's own hematopoietic stem cells through the chronic virus vector to produce normal hemoglobin and restore the function of red blood cells, which significantly reduces the blood transfusion needs of the patient. Under the ideal situation, the patient's patient There is no need to perform blood transfusion treatment.
According to Bloomberg, for most of patients with β-thalassemia who need to rely on blood transfusion most of the time, this therapy has "changed the rules of the game."
However, the FDA aspect also reminded that this treatment has potential risks that cause blood cancer. Although no cases have been seen in the study of ZyNTEGLO, patients receiving ZyNTEGLO treatment should be monitored for at least 15 years. During Zynteglo's administration, patients' superfluous reactions and platelet reduction and bleeding should also be monitored.

History is the most expensive
If there is no effect, you can get 80%expenses for reimbursement
According to reports, in the United States, according to the current nursing standards, the lifelong medical expenses of patients with blood transfusion dependencies are as high as US $ 6.4 million, and the average total medical expenses of each patient each year are 23 times that of ordinary people. According to Blue Bird Bio estimation, about 1,300 to 1500 people in the United States suffer from β-thalassemia.
Zynteglo is priced at US $ 2.8 million in the United States, and when it was approved in May 2019, the therapy was priced at 1.575 million euros (at the exchange rate at the time, about 1.8 million US dollars). Although the price in the European Union was much cheaper at that time, the commercialization process of ZyNTEGLO in Europe also faced many obstacles. In April 2021, Blue Bird Bio failed to reach a price agreement with the German government, and decided to temporarily withdraw the gene therapy Zynteglo out of the German market. Since then, the company has gradually withdrawn from the European market.
For the pricing of ZyNTEGLO in the US market, FDA believes that its price is the scope of cost -effectiveness in the independent review team.
According to reports, the payment model launched by Blue Bird in the United States is a one -time prepaid plus 80%of the risk sharing. If the patient fails to keep blood from blood transfusion within two years after infusion, commercial institutions such as patients or insurance will receive 80%of the cost reimbursement.
Blue Bird Biological said that about 70%-75%of β-neate anemia patients who relied on blood transfusion to enjoy commercial insurance. Subsidy agency cooperation. ZyNTEGLO will be officially listed in the United States in the fourth quarter of this year. Due to the cost of collecting cells to intravenous injection of 70 to 90 days, it is expected that formal treatment will be launched in the first quarter of 2023.
Bluebird creatures are expected to "rebirth"?

In the 1970s, the concept of gene therapy was proposed for the first time. By the 1990s, the clinical trials of genetic treatment in the United States were in full swing. In 1995, the first clinical case of successful gene therapy appeared in the United States, and gene therapy began to attract much attention.
Bluebird creatures were born in this wave. In 1992, two researchers at the Massachusetts Institute of Technology founded the predecessor of the blue bird creature Genetix Pharmaceuticals.
In the following decades, the development of genetic technology has gradually matured, and biotechnology companies have become more and more popular in the capital market. Bluebird creatures pursued by victory. In 2013, Nasdaq was landed. Before listing, the pricing was $ 17 per share. On the day of listing, it rose 50%, close to $ 26 per share. By 2018, the company's stock price rose to the highest point -$ 231/share, and at that time, the blue bird creature had no products to be officially launched.
Until 2019, the genetic therapy of Blue Bird Biological ZyNTEGLO was approved to be listed in the EU, but after encountering major setbacks, it withdrew from the area in 2021. Subsequently, Skysona, a genetic therapy for brain adrenal chiroprane malnutrition, also evacuated from the European market.
House seemingly endless rain. In May 2020, the CAR-T therapy BB2121 jointly developed by Blue Bird Biological and BMS for patients with refractory multi-myeloma, which was delayed by the FDA due to insufficient data. In November of the same year, LENTIGLOBIN's listing applications for Bluebird Biology for sickle -like cell anemia (SCD) were also postponed for a whole year.
After experiencing many setbacks, the blue bird creature began to go downhill, and its stock price went down all the way. As of the closing of US stocks on Thursday, its stock price plummeted 97.5%compared with the peak in 2018.
The reporter consulted the financial reports of Blue Bird Bio in recent years and found that in 2019, 2020, and 2021, the company's net loss reached US $ 790 million, $ 620 million, and $ 820 million, respectively. Blue Bird Biological Report in 2021 shows that the company has only about $ 442 million in available cash and related mobile assets.
The approval of ZyNTEGLO in the United States may help revitalize the business of blue bird creatures. Bloomberg analyst Marc Engelsgjerd wrote in a report, "After experiencing European failures and delays in the United States, the license (for the blue bird creature) provides a way to obtain considerable income."
According to the 2021 financial report of the Blue Bird Biology, the three gene therapy of Blue Bird Bio -Zynteglo, Skysona and Lentiglobin will usher in a new milestone, and it is expected to bring new revenue growth to it. At present, Zynteglo has taken an important step to the market. The company believes that SKYSONA is also expected to be approved and obtained priority review in 2022. At the same time, Lentiglobin is expected to submit a license application in the first quarter of next year.
Chinese enterprises are also developing local poverty -founder therapy

β-Mediterranean anemia is popular all over the world. Similarly, the number of poverty-stricken patients in China is also increasing.
The data of the "Chinese Mediterranean Anemia Blue Book (2020)" published in May 2021 shows that the poverty -stricken infrastructure carrier accounts for about 345 million people in the world, while there are about 30 million poverty -founder carriers in China. The number of people is about 33,000, and it is increasing at about 10 % per year.
Due to the high cost of blood resources and iron chelating, only part of Chinese poor patients can maintain blood transfusion and regulate the treatment of iron, and the survival rate of local poverty -stricken patients is significantly lower than that of developed countries.
At present, Chinese companies are also developing the treatment of poor gene therapy.
According to First Finance, on August 16 this year, Shanghai Bangyao Biotechnology Co., Ltd. (hereinafter referred to as "Bangyao Biological") announced that its gene therapy product "BRL-101 autologous hematopoietic ancestor for blood transfusion dependent β-thalassemia The clinical trial application of cell injection "officially obtained the approval of the China Pharmaceutical Supervision Bureau Pharmaceutical Review Center on the day.
Although the BRL-101 of Bangyao Bio and ZyNTEGLO of Blue Bird Biological belong to genetic therapy products, the two are still different. The former belongs to gene editing therapy products, and the latter belongs to the slow virus gene therapy product.
Bangyao Biological said that in the current traditional therapy of the ground, hematopoietic stem cell transplantation is the only way to cure β-thalassemia, but it is expensive and difficult to match. Only a small number of patients can get transplantation. The hematopoietic stem cells are returned to the patient's body after genetic correction, which can solve the problem of insufficient source of hematopoietic stem cells and difficulty in matching. The progress of the CRISPR gene editing technology provides the possibility of this treatment strategy. BRL-101 is a gene therapy product developed based on the company's independently developed hematopoietic stem cell platform. The platform mainly uses the gene editing system to generate the hematopoietic stem cells of the patient. And differentiation and reconstruction to modify the cell group, thereby achieving the purpose of treating blood system diseases. Bangyao Bio also stated that compared with other β-macromiolachen therapy tens of millions, the company's hematopoietic stem cell gene therapy is more efficient, convenient and secure, with good targeted, high safety, wide range of effects, treatment, treatment The advantages such as significant effects can be treated with a lifetime healing, and the cost can be greatly reduced, which is expected to become a therapy that benefits the public more.
Before the Bangyao Biological BRL-101 announced the clinical clinical, in January 2021, Boya's research product ET-01 was also approved in China for clinical trials in China due to the Blogen's dependent β thalassemia. Similarly, ET-01 also belongs to gene editing therapy products.
A little more news
- END -
Let the Pomegranate gene library go to the world

——In Shaanxi Pomegranate Industry Technical System Working Conference SideFrom J...
The Brazilian Central Bank raised interest rates 11 consecutive times to suppress inflation
The Brazilian Central Bank recently announced that the benchmark interest rate raised the benchmark interest rate from 12.75%to 13.25%. This is the 11th continuous increase in interest rates since Mar