Value broke out, East China Pharmaceutical "Three Driving Carriage" ushered in a high time of growth
Author:Kenji Bureau Time:2022.07.01
East China Pharmaceutical has been favorable recently.
On the evening of June 30, East China Pharmaceutical announced that the clinical trial application of Smeglugan injection in the injection of Smeglugan has been approved by the State Drug Administration; not long ago, East China Pharmaceutical and the well -known enterprise in the Middle East Julphar reached a strategic cooperation to achieve the "Liculu peptide injection product" Go to the sea "Middle East and North Africa.
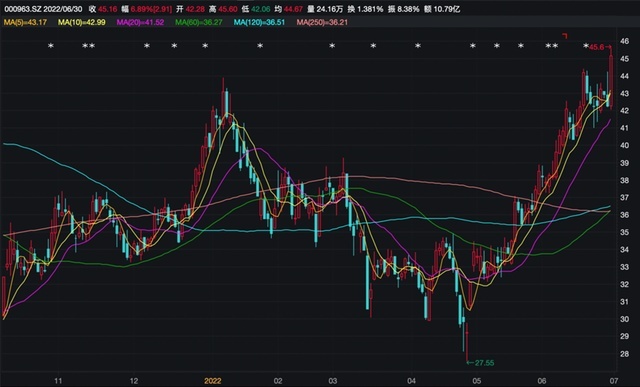
The confidence of these good news boosted the market. Since the release of 2021 and the first quarterly report, East China pharmaceutical stock price has risen steadily, breaking through the 60 -day season, the 120 -day semi -annual line, and the 250 -day annual line. On June 30, the stock price rushed quickly after the opening of East China Pharmaceuticals, which once rose by more than 7%, up to 45.60 yuan, a new year high, and the market value was approaching 80 billion yuan. As of the close, the East China Pharmaceutical Newspaper was 45.16 yuan, an increase of 6.89%, which has increased by 63.92%from the near 60 days.
The company's latest quarterly report shows that the two major public fundraising funds of China -Europe Medical and Zhao Bei, a subsidiary of Zhao Bei, and the well -known buyers such as Gao Yi Xiaofeng Fund of "Top Flowing Private Equity", appeared in the top ten shareholders of Huadong Pharmaceutical.
In addition, a number of seller agencies are optimistic about the future trend of East China Pharmaceutical's stock price and give a buy rating. Among them, CICC gives a target price of 47.8 yuan, and Guangfa Securities will rise to 50.1 yuan.
Compared with other companies in the pharmaceutical sector, when East China Pharmaceutical faced the transformation pain brought by the industry upgrade, the ancestors jumped out of the quagmire. The three major sectors of medical aesthetics, industrial microorganisms and innovation medicines all overcome difficulties and coordinate layouts. Through independent research and development, external cooperation, and License-in, we will deepen the layout of medical beauty and innovative drug business to drive medical beauty, industrial microorganisms, and innovative drugs. " "Three -driving carriage" developed a characteristic transformation and upgrade path.
Thanks to the forward -looking strategic layout, the strategic planning of the initiative, and the firmness of the execution, East China Medicine repeatedly repeatedly eliminated the passive elimination against the wind to become a nirvana of innovation.
Explosive products drive medical beauty super expected growth top pipeline casting medical faucet
The medical beauty sector is the core strategic field of East China Pharmaceutical Health Industry. With the gradual relaxation of overseas epidemic control measures, the Asia -Pacific market sales have been strongly promoted.
The overseas medical beauty business shows a rapid recovery trend, and the domestic medical beauty business has gradually returned to the right track. The performance of the medical beauty sector is expected to achieve a new round of outbreak.
As a leader in the medical beauty industry, East China Medicine fully integrates global medical beauty resources, enriches the quality of medical beauty pipelines, continuously expands the company's medical beauty innovation product pipeline, and introduces international first -class and high -tech gold -containing medical products to China one after another.
According to public information, the East China Pharmaceutical Medical Aesthetic sector has 36 high -end products of "minimally invasive+non -invasive" medical beauty, of which more than 20 products have been listed at home and abroad, more than ten models of global innovation products have been studied, and the product portfolio covers coverage. The mainstream medical beauty fields such as filling, buried threads, skin management, and body shaping have formed a comprehensive product cluster, and the number of products and coverage areas have ranked among the forefront of the industry. A variety of potential products are expected to be listed and sold at home and abroad after 2022, which will bring new growth momentum to the company's global medical beauty business.
In terms of sales, East China Pharmaceutical adheres to its own self -employment model. Relying on the company's professional clinical registration and marketing promotion team, it helps the rapid commercialization of international high -quality products, promote the continuous optimization of product structure, and gradually realize the company's strategic layout of the company's medical beauty.
East China Pharmaceutical Star Product Ellansé® Yiyan Shi® (Girls Needle) was officially launched in China in August 2021, which has attracted high attention in the domestic medical and beauty industry and the favor of beauty seekers. 100 million, with multiple advantages such as instant filling, long -term maintenance, and natural metabolism, became the "regeneration era" leader in the field of medical beauty injection.
Caitong Securities believes that the differences in pricing, channels, and brand tone between different medical and beauty products on the market are large. Differentiated product positioning and excellent marketing promotion capabilities can greatly improve the performance of the medical beauty product market.
According to the data, the marketing and sales teams of girls' needles and sales teams and main members are from top medical beauty and cosmetics companies such as Gaodemei, Eljian and L'Oreal. The first quarterly report of 2022 shows that the wholly -owned subsidiary Sinclair commercialization network has covered the world's major medical aesthetic markets. The products are sold to more than 80 countries and regions. The income was 3104 million pounds (about 260 million yuan), an increase of 163.1%year -on -year, the highest level in a single quarter, a significant losses year -on -year, and the first time in history achieved operating profit.
Domestic Xincari Aesthetics has actively expanded the coverage of cooperative hospitals and product promotion. At present, the number of cooperation hospitals has exceeded 400, and the number of training certification doctors exceeds 700. According to Caitong Securities, Girls' Needle is expected to achieve a revenue of 60,000 to 700 million yuan in 2022. In the future, it is expected to become the first 1 billion-level heavy variety in East China Medical and Medical Aesthetics and even the domestic medical beauty market.
It is worth mentioning that in May 2022, East China Medicine introduced EMA Aesthetics Energy Source Energy Source equipment Préime Dermafacial. This is a multi -functional facial skin management platform that integrates spiral vacuum, micro -crystal grinding skin, microcirculation, radio frequency, and ultrasound. And listed in China in 2023. Préime Dermafacial similar products -the product "HydraFacial" of the American listed company Beauty Health has become one of the hottest deep cleaning and maintenance high -end medical beauty projects since its launch. In 2021, the "Hai Feixiu" product realized a $ 260 million operating income, an increase of 118.4%year -on -year.
By comparison of product functions, the Préime Dermafacial introduced by East China Pharmaceutical this time is more than Hai Feixiu. While satisfying facial cleaning care, it also has both micro -crystal grinding, micro -current, radio frequency, ultrasound technology. The effect is more powerful. Realize the effect of skin regeneration, skin smoothness, and skin firmness.
Source: East China Pharmaceutical Finance Report
In addition, East China Pharmaceutical introduced the cold -touch beauty in the United States R2 Corporation GLACIAL SPA® (F0) has officially entered the Chinese market. It adopts a new DTC model to carry out sales and sales in the first batch of pioneers in 5 major cities in China in March. Services have opened online "GLACIAL Flagship Stores" in Tmall Mall, and simultaneously open online institutions and online sales. The company is also actively promoting high -end hyaluronic acid products MAILI, buried line products Silhouette Instalift®, freezing and freckle removal equipment GLACIAL RX (F1), frozen fat -soluble product Cooltech Define, laser hair removal equipment, etc., officially opened up the domestic medical beauty business in China The volume process of the line.
The first quarterly report of 2022 shows that the total operating income of the East China Medical and Medicine Aesthetics section achieved 453 million yuan, an increase of 226.8%year -on -year by comparable caliber (excluding East China Ningbo). Huatai Securities believes that the major varieties of East China Medicine will be approved one after another since 2022. The company has ranked among the domestic medical beauty. In 2025, it is expected to achieve nearly 5 billion yuan in revenue.
Industrial microorganisms thick hair, welcome highlights, innovative pills, heavy products are ready to be listed on the market
When the generic drug industry encountered anti -winds, East China Pharmaceuticals made an industrial microorganism development strategy of "exploring new blue oceans of biotechnology and layout of East China Pharmaceuticals".
In the past two years, East China Medicine has introduced the domain research and development team to continuously enrich the product pipeline, improve all technical links in the biological engineering technology industry chain, while continuously developing a broader biotechnology application scenario, focusing on special functional chemistry chemistry Products, pharmaceuticals and high -end intermediates, large health and medical raw materials, biomedical materials and enzymes, and microbial innovation drugs are committed to creating a "industrialized, large -scale, and international" industrial microbial industry cluster.
East China Pharmaceutical has a deep industrial foundation in the field of industrial microorganisms. It has successfully developed and manufactured a variety of microbial drugs, and built a key technical system for the development and production of microbial products. The scale and technological level of the existing microbial fermentation products are at the leading level in the industry.
According to the financial report, at present, East China Pharmaceutical's research and development capabilities in the industrial microbes have covered the various stages of microbial engineering technology such as fungus construction, metabolic regulation, separation purification, enzyme catalysis, and synthetic modification. It accounts for 21%. In the past two years, it has obtained 23 authorized invention patents. In the review of 59 patents, the product research and development pipeline has nearly 100 research and development projects. In 2021, the total operating income of industrial microorganisms achieved 418 million yuan, an increase of 69.2%year -on -year. In the first quarter of this year, operating income increased by 99%year -on -year. The development momentum continued to improve.
On May 10, the National Development and Reform Commission released the "Fourteenth Five -Year Plan" Biological Economic Development Plan "stated that during the" Fourteenth Five -Year Plan "period, my country's biotechnology and biological industry accelerated development, and the biological economy became a strong driving force for promoting high -quality development. The construction of biological safety risk prevention and control and governance system has been continuously strengthened. Among them, synthetic biology has been mentioned many times. As an important part of East China Pharmaceutical Innovation and Transformation, Industrial Microbiology is expected to benefit from the improvement of the demand brought by the development of the industry and outline the second growth curve of the company's pharmaceutical manufacturing sector.
The Research Report of Xingye Securities shows that the industrial microbes will meet a new round of market opportunities in the future, and it is expected to contribute considerable cash flow to East China Medicine.
In 2020, the number and amount of investment and financing events in the global synthetic biology field set a historical record, from 189 and US $ 5.66 billion, respectively. According to the synthetic biology innovation platform "Synbiobeta" survey, the investment in the first half of 2021 reached 8.9 billion US dollars, an increase of nearly tripled in the first half of 2020. Asia's synthetic biology companies have fewer investment and financing, but the average transaction value has reached $ 9.6 million.
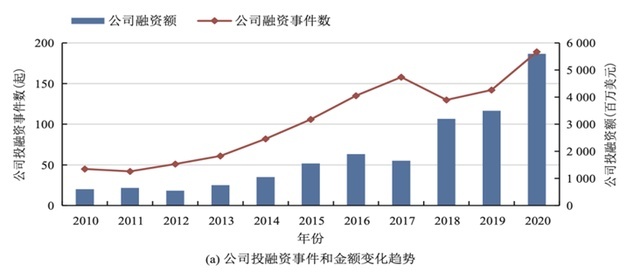
Tianfeng Securities pointed out that the 2021Q3 quarterly historic high -quarter financing high -quarter financing high -quarter financing high, the investment amount is as high as US $ 6.1 billion. From the historical data point of view, it has become the most prosperous year in the field of synthetic biology in 2021. It is expected to open synthetic biology. The first year of study and development. East China Pharmaceutical adheres to the corporate philosophy of "based on scientific research and patient -centered", continues to increase investment in R & D, and actively promotes the clinical research of research and innovation medicines, key biological types of substances East China Pharmaceutical Global R & D Ecosystem.
In the field of ADC, East China Pharmaceuticals continued to increase differentiation. HDM2002 (Mirvetuximab) is the world's first global ADC in the world's first global ADC for the world's first global ADC for the treatment of FRα high -expressed platinum -resistant ovarian cancer. In March of this year, Immunogen announced the submission of the biological product license application (BLA) of the product to the US FDA, and obtained the FDA acceptance and was granted priority review qualifications in May. The target date is November 28, 2022.
In February 2022, the company also launched equity investment and product cooperation with the global emerging technology company Heidelberg Pharma, the world's global technology companies, to further enrich East China Pharmaceutical's unique ADC global R & D ecosystem. According to the latest investor research records disclosed by East China Medicine, the German Federal Financial Regulatory Authority (Bafin) has approved the exemption of the company's public offer to Heidelbergpharma; the company has also obtained the German Federal Economic Affairs and Climate Action Department (BMWK). No objection proof; the approval or filing of relevant overseas investment in China in this transaction is being promoted as planned.
It is worth mentioning that at the recently ended 2022 ASCO conference, the first and third ADC new drugs ENHERTU shined. The results of the Destiny-Breast04 clinical trials that treat HER2 low-expression advanced breast cancer are exciting. Researchers believe that the Destiny-Breast04 result confirmed for the first time that ADC targeted therapy for HER2 can improve the pre-pre-survival of HER2 low-expression patients. The study also reminded that the use of HER2 targeted therapy for HER2 -negative patients may also improve benefits, and clinical practice of advanced breast cancer may be rewritten.
ENHERTU's success has greatly stimulated the enthusiasm of the ADC drug market. There are rumors of foreign media that SEAGEN, a pioneer in the ADC sector in Merck (Merck) in the United States. If this transaction is finally reached, the valuation of Seagen may be about $ 28 billion. ADC has become a place where the next soldier is involved.
In addition, Nicinna (Agline tablets) have been responsible for the promotion of the Chinese market by the East China team of China and the United States, and the sales situation in the process of marketing shows a better growth trend. As the epidemic in Shanghai improves, Nicina (Agleine tablets) is expected to further volume to complete the established sales target this year, and it has a positive effect on the competitiveness and performance growth of the company's diabetic product pipelines. The company's exclusive agent's 藿 软 soft capsule has also officially begun to supply to major hospitals and DTP pharmacies across the country. Epimedium Soft Capsule is an innovative small molecular immunomotive agent. Cell carcinoma.
East China Pharmaceutical Licula Peptide injection (diabetes indication) is the first domestic drug to submit a listing application. At present, drug registration and verification have been completed. It is expected to be approved to be listed in China by the end of 2022. Its weight loss indications are actively applying for listing applications and are expected to be listed next year. In the field of weight loss indications, there are currently no GLP-1 inhibitors in China, including the original drugs, which are approved by the original research drugs. East China Pharmaceutical is expected to get the top spot.
East China Pharmaceuticals has brought unprecedented market opportunities to the growth of the pharmaceutical industry in the field of innovative medicines.
East China Pharmaceutical's continued deep cultivation in the three major sections of medical beauty, industrial microorganisms, and pharmaceutical industry has consolidated the position of its industry leaders. The strong value of "driving troiders" has highlighted its climate, and it will establish a growth of future global performance maps. Sex, lead East China Pharmaceutical's cross -transformation cycle to open a new chapter!
#East China Medicine (SZ000963)###
- END -
[Follow] Reasonable rents!Eight departments issued notice
recentlyThe Ministry of Housing and Urban -Rural Development and the National Deve...
Grass -roots party representative Wang Weifeng: Dendrobium has made a large industry
