Break the monopoly independent research and development precision ® porous surgery robot to complete the gynecological clinical admission group
Author:Henan Daily Rural Version Time:2022.07.06
On July 2, 2022, it was learned from the press conference of the gynecological clinical progress of the MP1000 System of the MP1000 System of the Wing -porous surgical robot that the precise ® porous surgical robot independently developed in my country has completed the registered clinical group of gynecology. The previous surgical robot has already been Completing clinical clinical clinical clinical in urology, it is expected to completely break the situation where such robots have been monopolized by imported brands for a long time.
On the morning of the same day, the press conference was held at the same time in the form of online connection+offline venue in Beijing and Zhengzhou.

Picture: Professor Meng Yuanguang, Professor Ji Mei and Dr. Wang Jianchen connected
Domestic robots "shot" to complete gynecological high difficulty surgery
Since August 2021, Qixuan Medical® United Hospital of the People's Liberation Army and the First Affiliated Hospital of Zhengzhou University has carried out the multi -centered, random, single -blindness, parallel control gynecological clinical trials of the MP1000 system of the MP1000 system of the MP1000 system ", Under the guidance of Academician Lang Jinghe, the Chinese Academy of Engineering, led by Professor Meng Yuanguang of the General Hospital of the People's Liberation Army, and jointly carried out with Professor Ji Mei of the First Affiliated Hospital of Zhengzhou University.
On December 31, 2021, the precision ® porous surgery robot was completed in the General Hospital of the People's Liberation Army in the People's Liberation Army. On May 10 this year, the precision ® porous surgery robot completed a robot auxiliary ovarian cancer treatment in the hospital in the hospital.
At the press conference on the day, Dr. Wang Jianchen, CEO of Shenzhen Jingfeng Medical Technology Co., Ltd., used the three keywords with excitement, honor, and exceeding three keywords to express his feelings. The surgical robot research and development direction answered. He said that the completion of clinical experiments of gynecological registration is an important milestone for precision medical®, which is of great significance to rapidly improving the clinical application level of robotics in my country. Very honored to be able to complete gynecological clinical experiments with the top surgery experts in China, and jointly promote my country's strategic highland to move towards the operating robot industry. The precision robot is completely independent research and development, breaking the technical monopoly abroad of this field, a complete breakthrough in core technologies, and a new transcendence in key technical parameters. Based on the core technologies that are currently completely independent to provide better user experience, more innovative design, better image quality, more comprehensive functions and more characteristics of the user experience.
Dr. Wang Jianchen said that the precision ® porous surgery robot completed the clinical clinical clinical clinical of urology in 2021. Today, the gynecological registered clinical trial group was completed. It is working hard in the direction of the application of the whole department, and the faster and faster. Being able to apply the entire department is the goal of Qixian Medical®. The next step of precision Medical® is to push the company's best product to the market with the support of the entire department surgery and improve the penetration rate of domestic robotics surgery in minimally invasive surgery surgery. , Empower your doctor and benefit patients.
The import monopoly situation is expected to be broken
In 2014, Ji Mei, chief physician of the Gynecology of Zhengda First Affiliated Hospital, began to use surgical robots to serve patients. So far, more than 2,000 cases have been operated.
However, these operations are completed with the help of imported Da Vinci robots.
Since last year, Ji Mei has also joined the clinical trial of the porous surgical robot. This test surprised her.
"Domestic surgery robots can be compared with shoulder imported robots." Ji Mei said that the surgical robot provides a doctor with a technical operation level. In the future, when domestic robots officially enter the hospital, the price of robot surgery will undoubtedly be reduced. Bring another innovation of clinical technology.

Picture: Professor Ji Mei, First Affiliated Hospital of Zhengzhou University
Robotics auxiliary surgery is a breakthrough medical technology progress after laparoscopic surgery, while surgical robots are known as "the pearl in the crown of the robot industry". On the one hand, due to its intelligence, efficiency, and accuracy, it has broad application prospects in the field of surgery; on the other hand, it is limited to its high technical barriers, long research and development cycles, wide disciplines, large resource investment, etc. Participation of participants.
For a long time, internationally renowned surgical robot brands have occupied a monopoly position. High costs have not only limited the hospital's equipment configuration and the choice of patients, but also exacerbated the distribution of high -end medical resources in my country. Therefore, building a domestic independent brand and breaking the international monopoly of surgery robotics has become one of the core strategies for the development of the robot industry and intelligent medical equipment industry in my country.
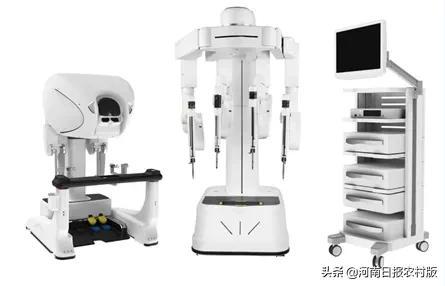
Bianfeng ® Pores and Pores Surgical Robot MP1000 System
The MP1000 of the MP1000 of the Fighting ® Multi -Pores is a robot auxiliary device. It can perform minimally invasive surgery through the application of robotic technology, imaging technology, and digital technology. It can be applied to urology, gynecological, general surgery and thoracic surgery. The precision ® porous surgery robot has now completed the registration clinical group of urology and gynecology, and launched the clinical enrollment in the general and thoracic surgery in May this year. The product has obtained national medicine in April this year. The Supervisory Bureau's Quick Examination Qualification (Green Channel) of Innovation Medical Device. (Li Haixu)
Review: Editor Sun Wei: Muzi
- END -
Furong State Comments | In these 800 standards, integration with China is in line with the world
A few days ago, the International Railway Alliance issued and implemented the High -speed Railway Design Infrastructure standard and High -speed Railway Design Power Supply standard formulated by
New Deal drives automobile market re -entered development fast lanes

Yu Dayong/PhotoOur reporter Yu DayongRecently, policies on vigorously promoting au...