The next day is completed!China's "fast" Car-T research selected asCo and EHA verbal reports
Author:Cancer Channel of the Medical Time:2022.07.20
*For medical professionals for reading reference
The result is surprising!
As the most top -level academic conference in the field of blood and oncology, the annual conference of the American Cancer Society (ASCO) and the European Society (EHA) in June each year will convey the world's latest and most heavier research progress. Each year, these two major meetings will accept excellent original research submission of the world, so the competition for oral reports is extremely fierce, and its results have attracted much attention.
Taking this year as an example, the ASCO Annual Conference has a total of 5,198 summary submission, while the EHA annual conference has a total of 2239 articles, of which 168 and 332 are related to multiple osteoma, respectively. In the end, there were only 13 multiple multi -myeloma abstracts selected in ASCO, and only 25 are selected into EHA. It is difficult to imagine.
At this year's ASCO Annual Conference and EHA Annual Meeting, my country has an oral reporting link selected by the original study based on Fast Car-T platform to treat multiple osteoma. Shanghai Long March Hospital) The BCMA/CD19 dual target of BCMA/CD19 dual targets reported by Professor Rhododendron of the Department of Hematology.
Why is this study favored by the two international conferences, ASCO and EHA? What are the profound impacts and significance of this study on the field of disease treatment? With these issues, Professor Rhododendron Dialogue in the medical world, trying to explain the "story" behind this verbal report.
Data "stunning": "Double excellence" of curative effect and security
The "protagonist" of this research-GC012F is a B cell mature antigen (BCMA) and CD19 dual-targeting auto-T products. It is based on FastCAR platform technology. It has the advantage of "completion of production the next day". -36 hours can be prepared. In the pre -clinical results of the pre -clinical and first human test, GC012F has continued to show excellent security and effectiveness. The current indications in research include multiple osteoma and B -cell non -Hodgkin lymphoma. In November 2021, GC012F was granted orphaned drug qualifications by the US Food and Drug Administration (FDA) for the treatment of multiple osteoma.
At the ASCO and EHA Annual Conference in June, Professor Rhododendron's research team provided the latest security and preliminary efficacy data of GC012F at a longer follow -up time.
This one-arm, open label, and multi-center researchers initiated test (IIT) were incorporated into 29 patients with R/R MM, 27-76, and gave a single infusion GC012F treatment.
Among these patients, the average number of past treatment lines is 5 lines (range 2-9), 89.3%are high-risk groups (HR-MSMART), and 8 cases are accompanied by EM diseases. ), 24 cases were difficult to cure for the final treatment, 3 cases were treated, and 9 cases were previously treated with anti -CD38 treatment, 27 cases were treated with immunomotive regulatory drugs (IMIDS), 26 PI were difficult to cure, and 26 cases of IMIDS were difficult to cure. "It can be seen from the clinical characteristics that some patients who have been incorporated in the past are patients with relatively difficult culminated myeloma." Professor Rhododendron said.
After 2-3 days of clearing gonorrheic chemotherapy (30 mg/m2/d, 300mg/m2/d flu/cy), GC012F infushed at 3 doses: 1x105/kg (2 cases), 2x105/kg (2x105/kg (2x105/kg ( 10 cases) and 3x105/kg (16 cases).
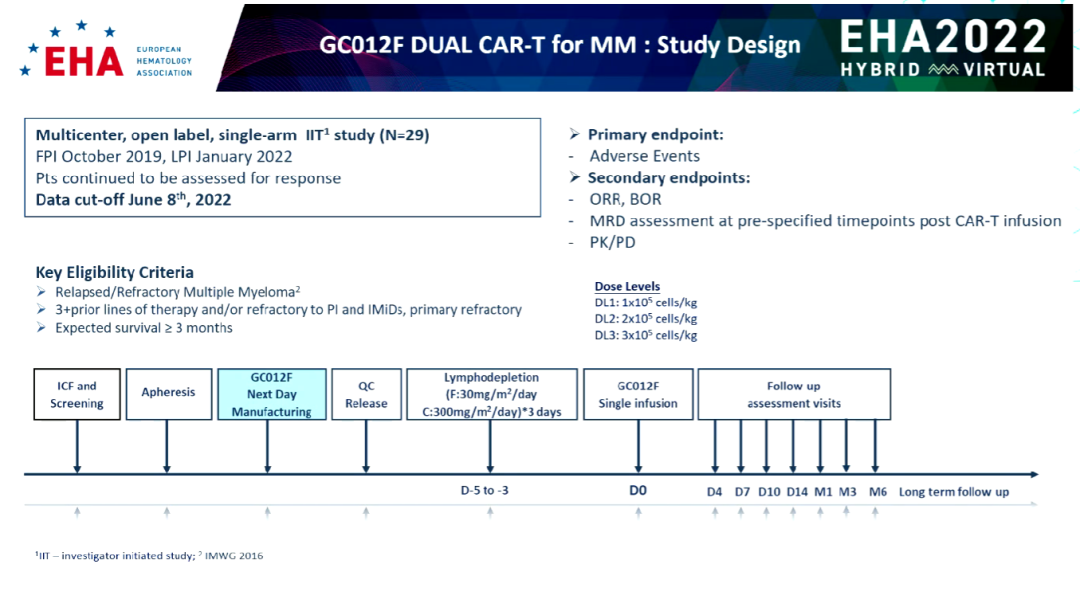
Figure 1: Research Design (Source: EHA Conference Report)
As of June 2022, the median follow-up time was 11 months (scope 4.9-34.5). The study found that:
① The total relief rate (ORR) reaches 93%, and the disease relief depth is good: the ORR of the 3 dosage groups is 100%(2/2), 80%(8/10), and 93.8%(15/16). 93%(Figure 2). Of the 29 patients, 27 patients with small residual lesions (104-106) of the minor residual lesions (104-106) were negative (100%) (Figure 3). During the 28th day of evaluation, 81.5%of the mRD negatives can be evaluated. 75%of patients (21/28) have reached MRD-SCR (MRD negative-strictly alleviated). At the end of the data, the medium -level disease relief time (MDOR) was 15.7 months.
Professor Rhododendron added: "If we use the 106 of European Europe as the MRD negative boundary value, 87.5%of patients are still in MRD negative after 1 year of treatment (Figure 4), which shows that the duration of the relief is also ideal. In addition, because, because, because of the because The last patient in the group is only 5 months of follow -up. As the follow -up time is extended, we believe that the time of the disease relief will definitely be better, and the result is even more exciting. "
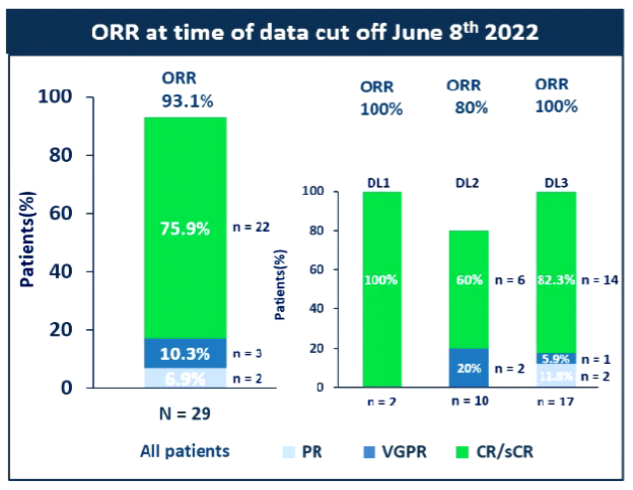
Figure 2: As of June 8, 2022, the total relief rate of the disease was 93.1%(Source: EHA Conference Report)
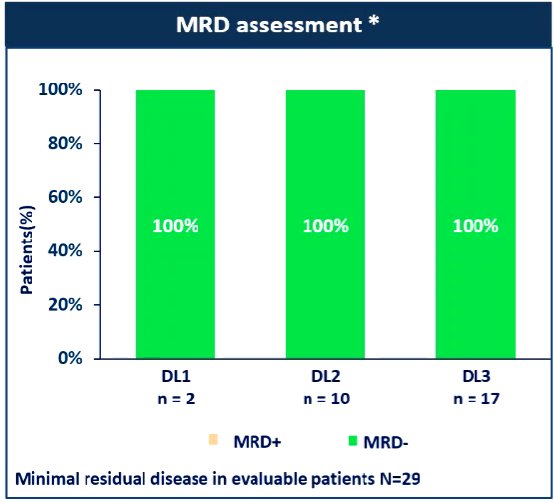
Figure 3: The MRD negative rate of 3 dosage groups reaches 100%(Source: EHA Conference Report)
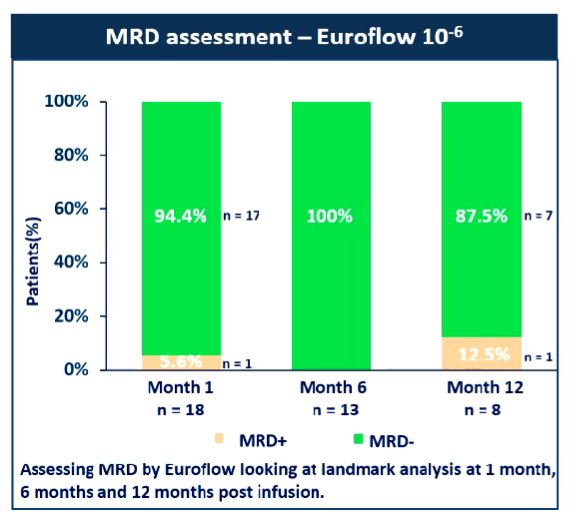
Figure 4: The MRD evaluation value is based on the EUROFlow 10-6. The MRD negative rate for 1 year after treatment is 87.5%(Source: EHA Conference Report) ② Good treatment safety: Of all 29 patients, 3 have not occurred in cytokine release synthesis The 23 CRS reactions of 23 cases (CRS) reacted level 1-2, and 2 patients reached level 3. The CRS of level 4/5 was not observed. The median duration of CRS is 3 days (range 1-8). At the same time, no neurological toxicity is observed.
③ The amplification state and duration of CAR-T cells are good: pharmacokinetics (PK) results show that there is no difference between dose levels. In general, CAR-T's median peak time (TMAX) is 10 days (range 8-14), and the medium peak copy number (CMAX) is 97009 (16011-374346) copy/UG DNA, the longest continuous continuity The time reaches 793 days (data deadline). The average AUC0-28 of the three dose group CAR-T geometry is 468863, 631540, and 581620 copied Day/μg Genomic DNA.
"Overall, GC012F has a very good state of expansion in the body of all patients and a longer duration." Professor Rhododendron commented.
Therefore, the research results show that the dual target CAR-T based on the new Fast Car platform is not only prepared for fast, but also the treatment of multiple osteoma patients with multiple osteoma has excellent depth and duration performance and good safety. "It should be said that this research has a preliminary exploration in the field of CAR-T in the future, especially in the field of double target CAR-T, provides a solid clinical data support." Professor Rhododenda Professor Rhododendron Say.
Source innovation: from basal to clinical clinical
Why can this study be attracted by international scholars? In addition to its excellent clinical effects, it is worth mentioning the innovation of therapy: dual targets and rapid production.
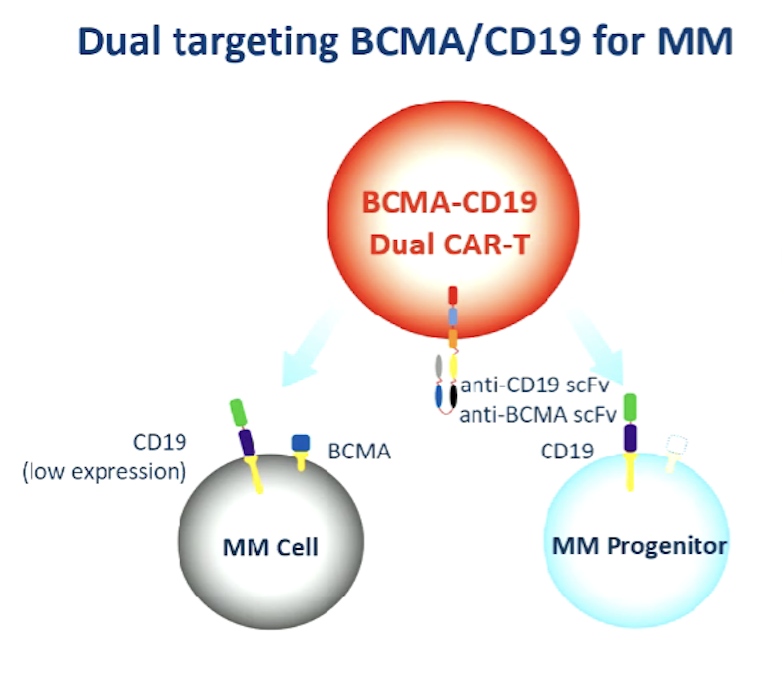
Professor Rhododendron explained this: "BCMA has a wide range of expression in the entire pulp cells, and CD19 can be expressed at the earlier ancestral cell stage. In the past The recurrence or progress of the patient is a problem that cannot be overcome. This time we combine the two targets during the CAR-T pre-preparation process and target different antigens at the same time. The problem reduces the decisive effect, thereby achieving deepening disease relief and more lasting treatment effects.
Figure 5: The principle diagram of BCMA/CD19 dual target CAR-T to treat multiple osteoma (Source: EHA Conference Report)
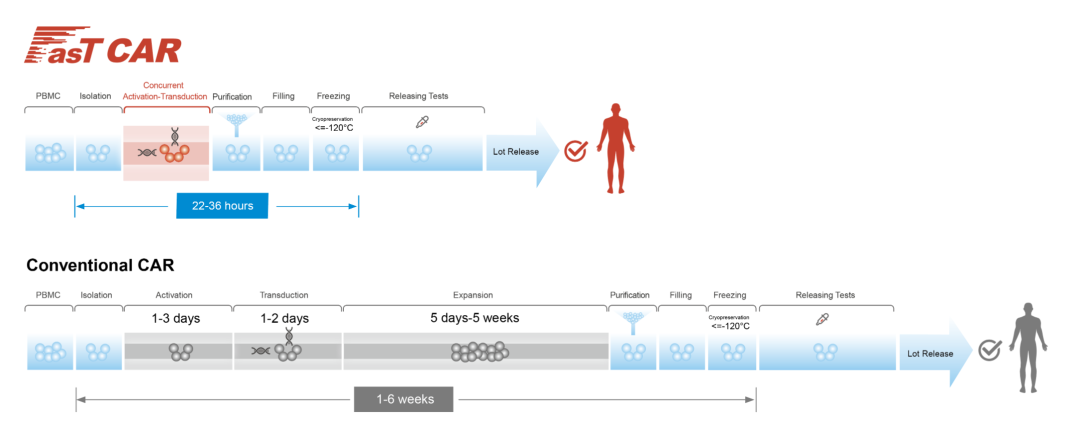
At the same time, based on Fast CAR's platform Car-T therapy, its preparation speed is very fast, and this detection process can save nearly 50%-70%than traditional CAR-T.
At present, the clinical study of patients with osteoma treated in CAR-T is patients with recurrence or difficulty in cure. Traditional CAR-T often takes a long time. During the waiting process, most patients have to receive bridge treatment. Bridge treatment patients also need to eluted. The use of fast preparation methods can save the waiting intermittent period on the one hand. At the same time, it is the dual target CAR-T in the body. The clinical effect seems to be more ideal and safe than the traditional CAR-T. Prospective CAR-T therapy. "
Figure 6: The basic principles of the FASTCAR platform and the comparison with the traditional CAR platform (Source: 亘 喜 图 网)
At present, recurrence/refractory multi -myeloma has always been the focus and focus of hematological tumors. In recent years, with the application and listing of many new drugs and therapy, there are more and more treatment choices for these patients. At the same time, many blood tumors clinical institutions are conducting corresponding clinical trials.
As an expert in the field of blood tumors, Professor Rhododendron also said: "In the past few years, the treatment concept of multiple osteomyoma has been updated rapidly. Properly monoclonal, Potumamine, Cafitzimi, XPO-1, BCL-2 inhibitors, BCMA dual-resistant, ADC drugs, etc., but how these drugs are matched and the treatment plan selection is important. Scientific issues. For patients with recurrence, it should be considered first in clinical trials to allow patients to have the opportunity to receive the latest drug treatment and save economic pressure. Patients with osteomyoma brings new opportunities to help many patients solve problems. We are also looking forward to being approved in China earlier. Benefit from Car-T therapy. "
Wonderful and presence: new progress of multiple osteomyoma
In addition to this study, at this year's ASCO and EHA annual conference, many other oral reports in the field of multi -bone marrowoma are also worthy of attention.
Professor Rhododendron concluded: "In terms of bone marrowoma treatment, researchers mainly focus on patients with two groups of first diagnosis and recurrence. In terms of treatment, the mainstream drugs or cornerstone schemes of osteoma treatment are updated, such as different combinations of drugs Strategy, follow-up for a longer period of follow-up, and the status of hematopoietic stem cell transplantation; on the other hand, new drugs, including double target antibodies, ADC, CAR-T and other new therapies. " Patients were included in the EHA Annual Meeting LBA (Late-Breaking ABSTRACT) Determination Research (LB2366) reported that NDMM patients received RVD (three therapies of Laibamine, boosamamisoni, and diceamonone). ) Safety and effectiveness. Studies have shown that, compared with patients who have not accepted ASCT, patients receiving ASCT have no significant differences in OS, but there is no progressive survival (PFS) significantly improved.
In the field of bispecidal antibody therapy in the recurrence refractory multiple osteoma (R/R MM), Majestec-1 research results show that whether patients with R/R MM have received anti-BCMA treatment in the past The CD3 bispecific antibody TECLISTAB treatment obtained depth and lasting relief, and the CRS adverse reactions are relatively controllable, most of which are at level 1-2. In addition, another BCMA/CD3 dual-resistant Elranatamab's related research Magnetismmm-3 also shows good overall efficiency and security.
Another new two-specific antibody drug Talquetama-related Monumental-1 research on the latest results of the GPRC5D/CD3 Research Assembly, the latest results of the Monumental-1 study may also have very good treatment prospects for RRMM, which had been treated with multi-line.
In addition, in terms of CAR-T therapy, in addition to the release of related real world research data on CAR-T products that have been listed in the United States, Chinese scholars also have heavy studies and announced, including human source CAR-T and targeting GPRC5D CAR-T, etc. Wait.
In addition, since the new antibody coupling (ADC) Belantamab Mafodotin has been approved to be listed in the United States, the EHA conference also shows the safety and effectiveness of its combined RD treatment NDMM patients.
"From the oral report of the international conference, it can be seen that the progress of multi -myeloma treatment is indeed very surprising, and it has also experienced the charm brought by new drugs and new therapies. And, we saw us at such a global event. The demonstrations of many studies by Chinese scholars reflect the development and progress of my country's bone marrowoma. We also look forward to the exhibition of more original research results on the international stage in the future, bringing surprises to the world, and adding bricks to the target of osteoma to cure. "Professor Dujuan finally said.
Expert Introduction
Professor Cuckoo
Director of the Department of Hematology at the Second Affiliated Hospital of the Naval Military Medical University (Shanghai Long March Hospital), director of the osteoma and lymphoma disease center of the Army. Chief physician, professor, doctoral supervisor. He has obtained a dual doctorate degree in medical and Southeast University of Medicine and Southeast University in Germany. He has been selected as the Shanghai Pujiang Talent Program, the eighth eighth youth science and technology talents in Shanghai, the "leading navigation" talent in the deep blue talent project, and obtained the "National Model of the People (Hematoma Tumor) Youth" in 2021. He is a member of the Expert Committee of the International Osmispermoma Working Group, a member of the Asian Osmisotoma Working Network (AMN), a member of the National Youth of the Hematology Branch of the Chinese Medical Association, and a member of the Multi -Osmisperteoma Professional Committee of the Chinese Medical Association Hematology Branch. At the same time, he also served as experts, writing or participating in many domestic and external osteoma -related guidelines.

He has long been committed to the clinical and scientific research of hematological tumors for a long time, and cultivates carefully and breaks through innovation. Focusing on in-depth exploration of targeted therapy for multiple osteoma, CAR-T cell immunotherapy, rare plasma cell diseases, etc., focus on the transformation of clinical and basic research. It has undertaken more than 20 scientific research projects, including 5 National Natural Science Foundation of China and 2 IMF projects (IMF) projects. He has published more than 20 SCI papers with the first author or communication (common) authors, including Blood, Leukemia, HAematologa and other blood leaders (the highest influence of 22.113 in a single article).
The first release of this article: blood channel in the medical community
Author of this article: Yu Xiaosu
Review of this article: Rhododendron of Shanghai Long March Hospital
Editor in charge: Sweet
- END -
There are 7 performances of "mature friendship" that reminds you of?

Professor William Rawlins had a survey on friendship and found that those who ente...
Zhang Baoyuan Incident: The generous sad song before dawn

Zhoukou Daily · Zhou Dao client reporter Wang Jinchun Wang Jicheng Gao Hongchi Ga...