Debeli: Dual Iopher nobles 丨 Exhibition Volume
Author:Return Time:2022.06.26
French physicist Louis Debro means that electrons have volatility in the doctoral papers of theoretical physics. At the invitation of Jiejie Lang Zhiwan, Einstein read the paper and wrote back to the letter, "The corner of the huge veil was opened." Debeli means that the light proposed by Einstein has the two phenomena of waves to all substances, thereby completely changing physics. For Debroy himself, physicists and nobles are also the "two phenomena" of his identity.

This article is authorized to be excerpted from Chapter 6 of the Quantum Biography, "Debeli: Two Iphorian Nobles" (CITIC Publishing House Nautilus, 2022.3), which is added by the editor. Go to the "Return to Park" public account and click "Read the original text" at the end of the article to buy this book. Click "Looking at" and publish your feelings to the message area. As of July 3, we will choose a message and give a book.
Written article 丨 Manjit Kumar
Translate 丨 Wang Qiaoqi
"Science is an old woman who is not afraid of mature men." His father said that. However, like his brother, he was seduced by science. Louis Victor Pierre Raymond Debeli was born in a French -level noble family. The family has always hoped that he would inherit the glory of his ancestors. The Debroy family originated in Piermont. Since the middle of the 17th century, he has been the general, politician and diplomat who has been the king of France since the middle of the 17th century, with a prominent status. To recognize their contributions, Louis XV was awarded a hereditary title of the Duke of the Duyi family in 1742. Victor Fransova, the son of the first generation of Debro's family, defeated the enemy of the Holy Roman Empire, and the empire emperor granted the title of Prince Prince to recognize his achievements. Since then, the descendants of Fransowa are either princes or county owners. Therefore, Debroy's young scientist will one day will become the German prince and Duke of France in the future. For a person who has made basic contributions to quantum physics, this family history seems a bit unnatural. The description of Einstein's contribution to Debro is "the first faint dawn of our worst physics mystery".
Brothers go to the road of physics
On August 15, 1892, Louis Debro was born in Diep. It was the smallest of the four children who did not die in the family. Debeli and his brothers and sisters were guided by private teachers in the ancestral mansion, which was also commensurate with their lofty status in society. When other boys of the same age could list the names of major steam engines at that time, Louis could already say the name of all ministers of the third Republic of France. In order to make the family, Louis will make a speech based on political reports in the newspaper. His sister Polyina later recalled that everyone soon felt that Luffy would "become an excellent politician" like the grandfather of the French Prime Minister. If their father died at the age of 14 (1906) at the age of 14 (1906), Louis may really become an excellent politician.
After his father died, Louis's brother -Morris, 31, became the master of a family. According to the family tradition, Morris followed the army, but he chose to play for the navy rather than the army. When studying at the Naval Academy, Morris's scientific achievements were very good. As a promising young officer, he found that the Navy was in a transition period for preparation for the 20th century. Considering Morris's interest in science, he participated in the construction of a reliable wireless communication system between ships. In 1902, Morris wrote his first paper with the theme of "wireless radio waves". This paper firmly left the Navy's determination to leave the Navy regardless of his father's opposition and completely put into the embrace of scientific research. In 1904, after 9 years of service, Morris officially withdrew from the Navy. Two years later, his father died, and Morris attacked himself to become the sixth Duke of Debroy, and had to shoulder a new responsibility.
At Morris' suggestion, Louis went to school to study. "I have experienced stress myself, so I have brought about the unhappy learning of young people. Therefore, I did not set a strict direction for my brother's academic settings, even if his indecisive indecisor sometimes makes me worry." Morris like this after nearly half a century later Written. Louis performed well in French, history, physics and philosophy, and did not care about mathematics and chemistry. Three years later, in 1909, 17 -year -old Louis officially graduated, and also obtained a degree in philosophy and a mathematics degree. One year before Louis graduated, Morris received a doctorate under the guidance of Paul Langzh Wan, French Academy, and established a laboratory in his house on Chadolion Street, Paris. Morris did not seek faculty in the university in order to engage in new walks, but founded a private laboratory, which helps to alleviate the disappointment caused by some family members because of his abandonment of military career transfer science.
Unlike Morris, Louis's career planning at the University of Paris was even more traditional. However, the aristocracy, only 20 years old at the time, quickly discovered that he could hardly raise any interest on critical research on the past text, information and documents. Morris later said that his brother was "not far from losing self -confidence." Part of the reason for this result is that working with Morris in the laboratory has made Louis's interest in physics rapidly. It turns out that the enthusiasm of my brother's enthusiasm for X -ray will be "contagious". However, Louis doubt whether he is capable of doing physical research, and a physical test failed to deepen his doubts. Louis has been thinking, is he destined to fail in physics? "The kind of lively and strenuousness of adolescence is completely disappeared! The chatter that shone shining with wisdom in childhood was annihilated under his deep thinking." Morris later remembered this when this interior brothers who could hardly recognize him were like this. Evaluation. According to his brother, Louis will become a "unruly and simple scholar" who does not like to leave his home. Louis first went abroad to October 1911, when he was 19 years old, and his destination was Brussels. After leaving the Navy, Morris became a respected scientist who specialized in X -ray physics. Morris readily accepted when he was invited to invite him to ensure that the first Solvi meeting was successfully held. Although this is just a management position, the opportunity to discuss quantum theory with characters such as Planck, Einstein and Lorenz is too tempting to refuse. The French have a lot of representatives at this meeting. Mrs. Curi, Ponkara, Piram, and Morris before attending the meeting.
Louis, who lives in the Metropolitan Hotel with all the participants, keeps a distance from these scientists, but only after they returned to the room and Morris told Louis that after the discussion of the quantum problem in a small room on the first floor of the hotel, the latter was the latter. Beginning with this new physics has a stronger interest. After the meeting was officially released, Louis read these materials carefully and determined to become a physicist. At that time, he had replaced historical textbooks with physics textbooks and obtained a scientific diploma in 1913, which is equivalent to a bachelor's degree in science. However, Louis must officially carry out his own scientific research plan after serving for one year. Although the Debrowe family and the three marshals in France have private relationships, Louis still joined a engineering company stationed outside Paris as a low second -class soldier. With the help of Morris, Louis quickly transferred to the wireless communication department. However, all the hope of quickly returning to physics research disappeared with the outbreak of the First World War. In the next 4 years, Louis was stationed under the Eiffel Tower as a radio engineer.
Is electrons volatility?
In August 1919, Louis finally left the army. He was resentful about his military career in the six years of his 6 years. At this time, Louis wanted to go along the way he had long chosen a long time ago. He got the help and encouragement of Morris, and repeated the research on X -rays and photoelectric effects in the latter's well -equipped laboratory. The two brothers discussed the experiments on how they were conducting for a long time. Morris reminded Louis's "educational value of experimental science" and "no value of scientific theory that has no facts support." Morris wrote a series of papers about X -ray absorption when thinking about the essence of electromagnetic radiation. The two brothers accepted the theory of fluctuations and particle theories of light to some extent, because none of them could not explain the diffraction, interference phenomenon and optoelectronic effects of light at the same time. In 1922, Einstein was invited to give lectures in Paris, but was a hostile reception because he stayed in Berlin during the first World War. That year, Debro wrote a paper that clearly used the optical quantum hypothesis. That is to say, when Connpton has not yet issued any public statement on his experiments, Louis has accepted the existence of "light atom". Waiting for the experimental data and experimental analysis of Electronic scattering X -ray, the American, the American, had learned how to get along with the strange wave -grain two phenomena. However, others just complained about half a joke at this time that in the future, the theory of fluctuations in the volatility of the light every Monday, Wednesday, and Friday, the two, four, and sixth religion particles theory.
"After thinking lonely for a long time," Debro said later, "In 1923, I suddenly had an idea: Einstein's discovery in 1905 should be generalized, and it will be expanded to all all to all to all to all. Material particles, especially electronics. "Debro boldly asked such a simple question: Since the light wave can show particle nature, can particles such as electrons show volatility? His answer is yes, because he found that if the electrons allocate a "virtual correlation wave" with a frequency of ν and wavelength λ, it can explain the accurate track position of the electron in the Bohl quantum atomic model. Electronics can only occupy the orbit that can accommodate its "virtual -related wave" integer wavelength.
In the hydrogen atomic model of Lutherford, electrons around the nucleus movement will continue to radiate energy and fall into the nucleus, and the entire atom will collapse. In order to avoid this theoretical dilemma, Bohr was forced to add a condition to this model in 1913 that he could not provide other reasons: the electrons that moved on the orbit around the atomic nucleus would not emit radiation. Debro's idea of seeing electronics as a station completely deviated from the thoughts of seeing electrons as particles surrounded by nuclear movements. The tight strings at both ends can easily produce a wave, such as violin strings and guitar strings. This type of string can produce various dwellings. The typical characteristics of such waves are that they are composed of integer times more than half -wave. The longest residence wavelength is twice the chord length; the second -length resident wave is composed of two semi -wave units, and the wavelength is equal to the physical length of the string; The wavelength is equal to 2/3 chord length; and so on. Such an integer twice wave sequence is the only allowed to appear in physical, and each of them has its own energy. Considering the relationship between wavelength and frequency, the above conclusions are equivalent to such a fact: After the guitar strings are dialed, they can only vibrate at a specific frequency starting from the basic tone (minimum frequency).
Debro realized that it was this "integrated" condition that limited the orbit occupied by the Bohr atomic model to the orbit where the perimeter was enough to form a bog. Different from the instrumental waves, there are no "tightening" at both ends of these electronics waves. The reason why they can form is because the perimeter of the track can accommodate the integer double -wave. Those orbit permeable places that cannot be accurately matched with the integer half -waves cannot occur in waves, so there is no fixed orbit.
Tightened string at the end of both ends
Electronic Waves in Quantum atoms
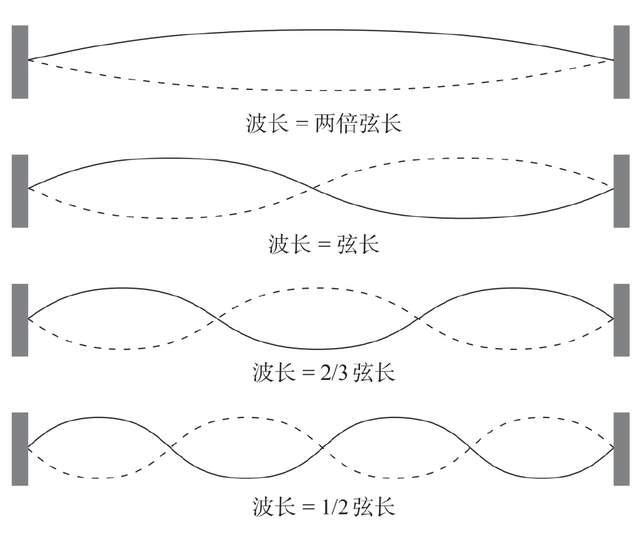
If the electrons are regarded as a particle around the waves around the atomic nucleus, it will not accelerate, and it will not continue to radiate energy. The last one is planted into the atomic nucleus and causes the entire atom to collapse. In order to save his quantum atomic model, Bohr was proven in the two phenomena theory of Dorbro's wave -grain two phenomenon theory. After the specific calculation of Debro, the main quantum N proposed by Bohr can only represent the orbit where the electronic waves exist around the hydrogen atomic nucleus. This is why there are no other electronic orbit in the Bohl model.
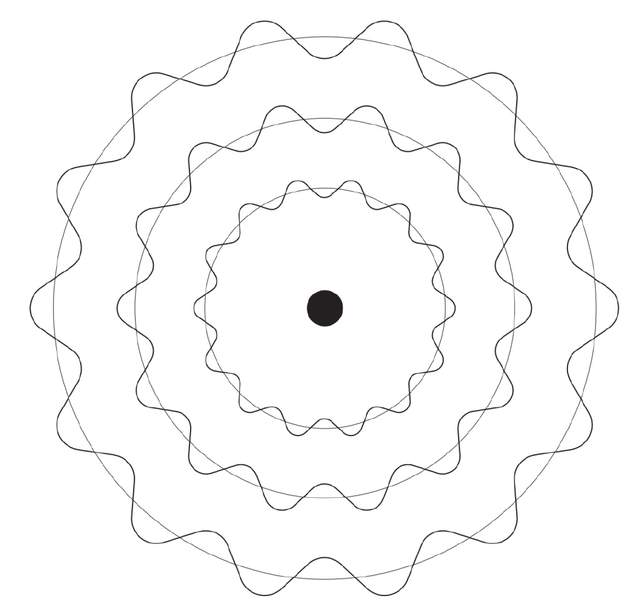
A corner of the huge veil opened
When Debeli meant in the fall of 1923 in the fall of 1923, why should people not immediately realize that all particles should be the two pixels of the particles as the two pixels. The essence of relationship. Is Debro said is it similar to surfingers on the cusp? Later, everyone confirmed that the explanation was incorrect. What Debroy expressed was actually the nature of electrons and all other particles as the photon: they all have two pixels. In the spring of 1924, Deblo intends to write his thoughts in the form of expansion and submit it as a doctoral dissertation. The necessary reception procedures and reviewers' review means that Delor's meaning will not start to post a doctoral defense until November 25 of that year. Three of the 4 judges are professors of the University of Somon. They are: the important role in testing Einstein's theoretical interpretation of the Brown Movement, Jean Piram. Outstanding physicist Charles Mogan, and famous mathematician Elli Jiadang. The last judge was invited from outside the school. He was Paul Langli, and only he was proficient in quantum physics and relativity. Before the formal submission of the paper, Delbro was looking for Lang Zhiwan first, and asked him to see how the paper concluded. Lang Zhiwan agreed, and later said to a colleague: "I now bring the dissertation of the younger brother of De Bro -Yi's family. The content is a bit far -fetched in my opinion."
The idea of Louis Debro's meaning may be a little whimsical, but Lang Wan did not immediately deny it. He also had to consult the opinion of another person. Lang Zhiwan knew that Einstein announced publicly in 1909 that the future radiation research association explained a combination of granularity and volatility. Compton's experiment made almost everyone convinced Einstein's light theory is correct. After all, anyway, this experiment looks like particles collide with electronics. Now, Debro is proposed that all substances have the binding of this wave particle. He even proposed a formula to connect the wavelength λ of the "particle" with its momentum P, that is, λ = H/P, where H is the Planck constant. Lang Zhiwan Xiang Debroy, a physicist as a noble, asked for a second copy of the paper and sent it to Einstein. "He opened a corner of the huge veil." Einstein said to Lang Wanwan.
For Lang Zhiwan and the other three judges, Einstein's evaluation is enough to explain the problem. They congratulated Duru Luo that "they learned to use superb skills while giving great efforts. Such efforts and skills were tried by physicists to overcome difficulties." Mo Gan later admitted that he "did not believe that the waves would be associated with material particles." The only thing Pilan can be sure is that De Bro is "very smart". As for others, he has no idea at all. With Einstein's support, Louis, 32, is no longer just a noble Louis Victor Piel Raymond Debro. PhD.
Unexpected experiments discovery
With the idea is just one aspect, but can this idea stand the test? In September 1923, Delbroy realized that if the material has the nature of the waves, then a beam of electrons will spread out like a beam of light -they should be diffracted. In a short paper written by Delbro, Debeli predicted: "A set of electrons through small holes will show the diffraction effect." He tried to persuade the outstanding experimental physicists working in his brother's private laboratory Test this idea, but failed to succeed. These skilled experiments were busy with other projects at the time, and they also thought that this experiment was too difficult to do. Louis felt that he felt that his brother Morris's "the importance of the two phenomena and the indispensable accuracy" of the radiation wave particles felt owed.
However, a young physicist at the University of Gostegen, Valter Elshaze, soon pointed out that if De Bro is right, only a simple crystal can make a beam on it on it. Electronic diffraction: Because the gap between the adjacent atoms in the crystal is enough to make the electrical objects reflect the volatility. "Young people, you sit on a gold mine." Einstein said to the latter after hearing the experiments proposed by Elshaze. In fact, Elshaze is not a gold mine, but a more precious thing: the Nobel Prize. However, like all the gold rush tide, if you want to gain something, you can't wait for too long. Unfortunately, El Shaoze did not put into practice in time, and the other two scientists took the lead in completing the experiment and announced the results, thereby holding the Nobel Prize in their hands.
Clinton Davidson, 34 years old, was studying the results of the 34 -year -old Clinton Davidson, who was a more well -known name of this institution. One strange thing happened in April 1925. A can of liquefied air exploded in the laboratory and broke Davidson's real vacuum tube with nickel targets. The air caused nickel rust. Davidson cleared the rust on the nickel by heating, but as a result, the tiny nickel crystal array became larger, which caused electronic diffraction. After Davidson continued the experiment, he soon realized that the experimental results were a bit different, but he did not realize that he had spread the electrons, so he just recorded all the experimental data and published it.
"After counting today, we will appear in Oxford. This sounds unlikely, right? We should have a wonderful time, dear Lotty. That will be our second honeymoon. Traveling, and it should be sweeter than the first time. "Davidson wrote in a letter to his wife in July 1926. The children stayed at home by their relatives. The Davidson couple could visit the UK before going to Oxford and the British Science Promotion Association to take a good rest -this is what they need. It was in Oxford that Davidson was shocked that some physicists believed that his experimental data proved the theory of a French nobleman. Prior to this, Davidson had never heard of Debro, nor did he hear that the two phenomena proposed by the latter could extend to all material. This situation is more than Davidson.
At that time, few people read the three short papers in Debro, because they published on the French journal "Compte Rendu". There are fewer people who know Dr. Dabro's thesis. After returning to New York, Davidson and his colleague Reast Ge immediately began to check whether electronics really diffracted in the experiment.
In January 1927, Based on the new experimental data, Davidson calculated the wavelength of the electrons after the diffraction, and found that the result was consistent with the dual theory of Dabro's waves. Decisive evidence of diffraction like waves. Davidson later acknowledged that the initial experiment was actually carried out after the experiments he did for the employer, and his employer was busy fighting with competitors at the time.
Max Knol and Ernst Rusca soon used electron volatility to invented electronic microscopes in 1931. No particles smaller than the white light of about 1/2 wavelength will absorb or reflect light, so ordinary microscope cannot observe these particles. However, electron microscope is only 1/100,000 because the electron wavelength is less than 1/100 000. In 1935, the first commercial electronic microscope was opened in England.
When Davidson and the end of the Ge ended in the experiment, British physicist George Peggi Tom Sun also conducted his experiments with an electronic beam in Aberdeen, Scotland. He also participated in the British Science Promotion Association, which was held in Oxford and Dorbro, was widely discussed. After the meeting, Tom Sun, who was interested in electronics, immediately began to detect the diffraction of electron through experiments. However, he did not use crystals, but instead used a special thin slice. The diffraction pattern generated on this thin piece was completely consistent with the theoretical prophecy of Debro. Therefore, the material does show the nature of the waves, spreading away in a vast space; at other times, it will show the nature of the particles and fix it in a position in the space.
Destiny is so amazing, the two phenomena of the material waves are deeply linked with the Tom Sun family. George Tom Sun and Davidson jointly won the 1937 Nobel Prize in Physics for confirming the volatility of electrons, and the former father's father was the J. J. Tomcho of the 1906 Nobel Prize in Physics because of the particle nature of the electrons. In this about 1/4 century, the development of quantum mechanics (from Planck's black body radiation law to Einstein's light quantum theory, from Bohr's quantum atomic model to Dabro's material wave particles. ) It is the product of the concept of quantum and classic physics, but their marriage is not happy. At least until 1925, this combination has begun to face increasing pressure. "The more successful the quantum theory, the more stupid it looks." Einstein wrote as early as May 1912. People urgently need a new theory, which is a new mechanics system describing the quantum world.
Steven Winberg, the Nobel Prize winner of the United States, said: "In the middle of the 1920s, the discovery of quantum mechanics was the most far -reaching theoretical revolution since the birth of modern physics in the 17th century." The physicists who played an important role in the revolution were very young at the time. People calling this time belonged to the years of "Knabenphysik" -the word means "boy physics".
↓↓ Go to the "Return to Park" public account and click "Read the original text" to buy at the end of the text
Special Note
1. Enter the "Boutique Column" at the bottom menu of the WeChat public account of the WeChat public account.
2. "Returne" provides a monthly retrieval article function. Pay attention to the public account and reply to the year+month for the four digits, such as "1903", you can obtain an index of articles in March 2019, and so on.

Copyright explanation: Welcome to repost personal. Any form of media or institutions shall not reprint and extract without authorization. Reprinted authorization, please contact the background within the WeChat public account of "Return to Pu".
- END -
A total of 100 good books | The season of harvest, a good book accompany you in summer

June is the season of harvest. The students have been planting for many years and ...
nice!The core landscapes such as Santan Yinyue enter the "Hangzhou West Lake Commemorative Coin", the global launch of the world

Zhejiang News Client reporter Quan Linxun Editor Wang JinshuaiI failed to throw Ha...