The longest MOS reaches 32.9 months, K -drug target free combination brings new ideas of "de -chemotherapy" of advanced head and neck cancer | ASCO 2022
Author:Cancer Channel of the Medical Time:2022.06.15
*For medical professionals for reading reference
The latest research on the advanced head and neck cancer of K medicine is released. The research details and clinical significance are explained in detail!
The annual Global Oncology Congress American Clinical Oncology Society (ASCO) Annual Conference was held online and offline from June 3 to 7, 2022. As an authoritative academic exchange event in the global tumor sector, this ASCO also brings cutting -edge research results in the field of head and neck cancer. One of them is called "A Phase II TRIAL TRIAL TRIALILIZUMAB and Cabozantinib in Patient (PTS) With recurrence head and note Squamous Cell Car-Cinoma (RMHNSCC) II, multi-centered multi-centered, and single-arm clinical studies on labels, and evaluated Paborizumab combined with Cabozantinib treatment recurrence/metastasis (R/M) head and neck squamous cell carcinoma ( HSNCC) patients' safety and tolerance, and successfully selected oral reports (Abstract number: 6008).
Figure 1. Screenshot of ASCO official website
"Immune+targeted" combined therapy is effective and safety among patients with recurrence/metastasis HNSCC? What are the dazzling things in this study? The "medical circles of tumor channels" are sorted as follows to readers.
Keynote-048 establishes a front-line position of immunotherapy, and K-drug further finds "new partners" in synergy to increase efficiency
Globally, more than 60%of HNSCC patients are local advanced (III/IV) at the initial diagnosis, and more than 50%of local advanced HNSCC will recur or transfer within 3 years [1-2]. Patients with the progress of the disease are poor prognosis and high probability of recurrence, and the quality of life is often seriously affected by the disease and repeated treatment.
As far as the treatment of R/M HNSCC is concerned, the 2022 edition of the "Chinese Clinical Oncology Society (CSCO) head and neck tumor diagnosis and treatment" (referred to as "CSCO Guide") pointed out that patients with local and/or neck recurring surgery still save sexual surgery. It is a commonly used treatment method, and for patients who have not received radiotherapy in the past, surgery+postoperative adjuvant radiotherapy is usually given; for patients with distant metastasis, the CSCO Guide Compared with the traditional Extreme solution, the combined chemotherapy scheme has carried out I-level recommendations for the Paborizumab+cisplatin scheme and Paborizhu single anti-single drug treatment (CPS ≥ 1) [3-4].
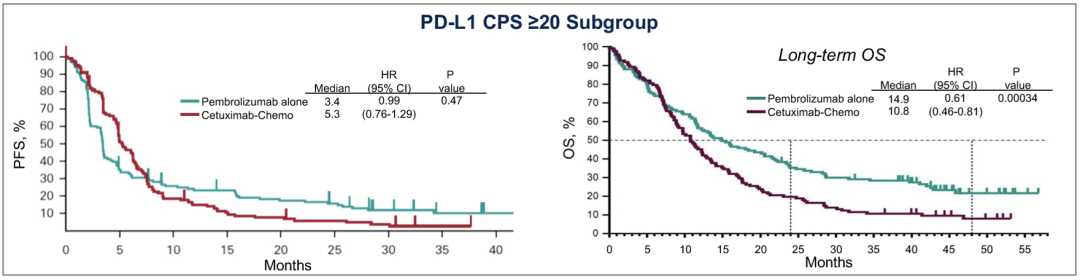
Figure 2. Keynote-048 Study PFS data and long-term OS data
Although the treatment plan based on Pabberzumab has significantly extended the survival of patients with R/M HNSCC, the prognosis of patients with R/M HNSCC still has room for further improvement. In addition, as clinicians and patients attach great importance to the quality of life, the exploration of clinical workers in recent years is gradually advancing.
Cabozantinib is a multi -receptor tyrosine kinase inhibitor (TKI) that targets MET and Vegfr2 at the same time. While inhibiting tumor growth and metastasis, it can also inhibit blood vessel production and has an immune regulatory effect. At present, CABOZANTINIB has been approved by the US Food and Drug Administration (FDA) for single drugs or combined with other drugs to treat kidney cancer, hepatocytal carcinoma, and thyroid cancer. Previous studies have shown that antemlodible -generating drugs can improve tumor micro -environment and use "synergistic efficiency" effects with immunotherapy [5]. The study of the summary number 6008 in the ASCO verbal report is a new attempt for "immune+antiovascular production" in the field of head -neck cancer.
PD-L1 high-expression patient OS is as high as 32.9 months.
Phase II studies have been included in patients with non -surgical resection. Patients with R/M HNSCC are measured according to the RECIST V1.1 standard. The number of past radiotherapy times is ≤1 times, and the expected life expectancy is 3 months. The ECOG score is 0 or 1. The patient was treated with Paborzab (200mg Q3W intravenous injection) combined with Cabozantinib (40mg QD oral). The main endpoint is the safety and tolerance of patients and an objective relief rate (ORR). Study preset Paborzumab combined with Katabitinib therapy R/M HSNCC Orr will be increased to ≥35 % (Paborizumab therapy for the treatment of 18 %).
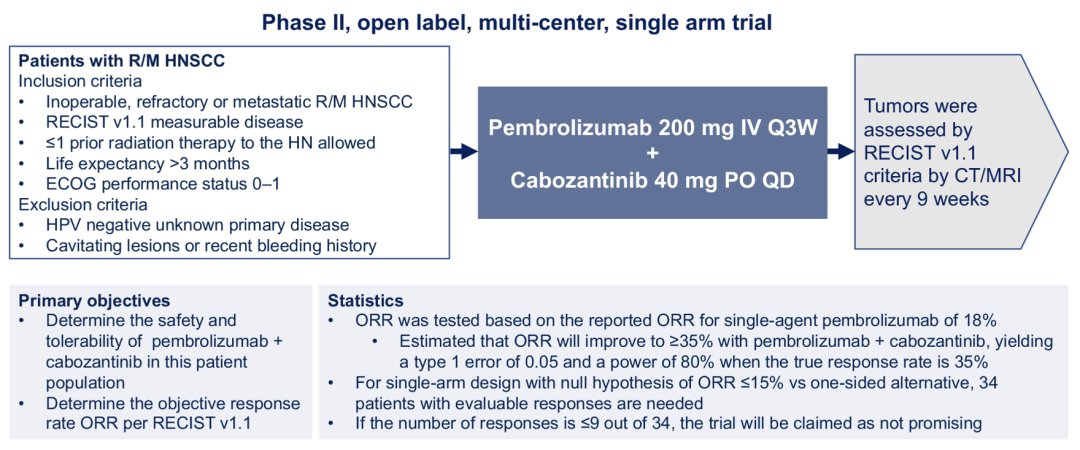
Figure 3. ASCO #6008 Research Design
The 50 patients who were screened were admitted to 36 patients, of which 33 were appraisal tumor relief. The average age is 62 years (54-67), most of which are patients with PD-L1 CPS ≥ 1 (50 % of patients PD-L1 CPS ≥20; 44 % of patients with PD-L1 CPS1-19; 6 % of patients with patients PD-L1 CPS <1).
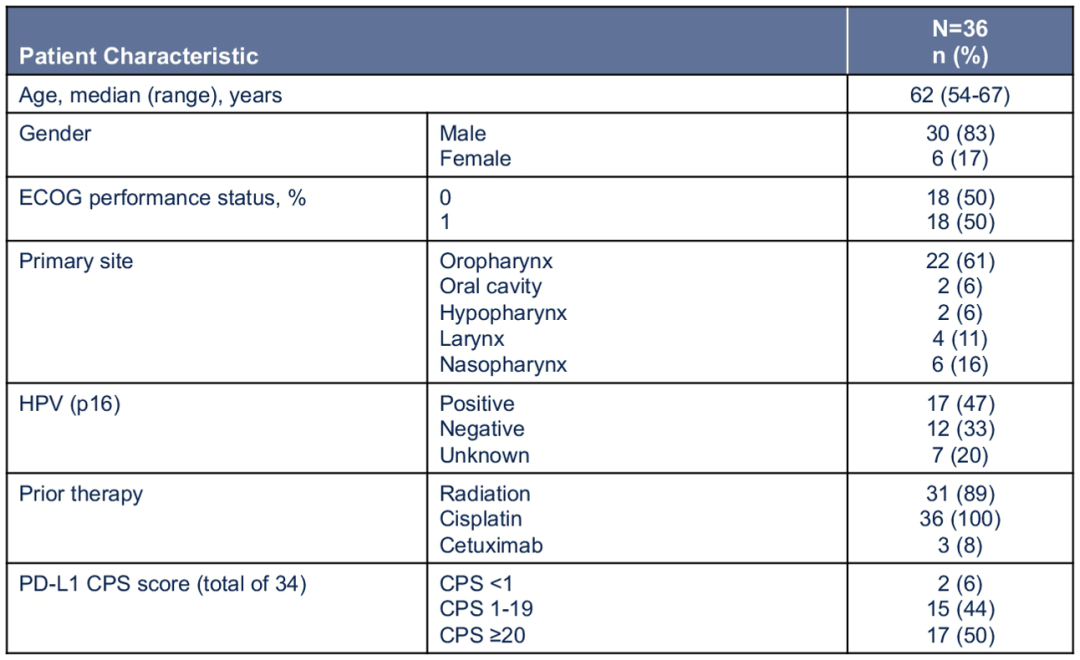
Figure 4. ASCO #6008 Study patient baseline characteristics
The results showed that in terms of efficacy, Paborizumab and Cabozantinib obtained the research and also reached the preset ORR main endpoint (ORR ≥ 35%). Among the 33 patients who can be approved, the overall ORR is as high as 54 % and the clinical benefits rate is as high as 91 %. Figure 5. ASCO #6008 Research Main End ORR data
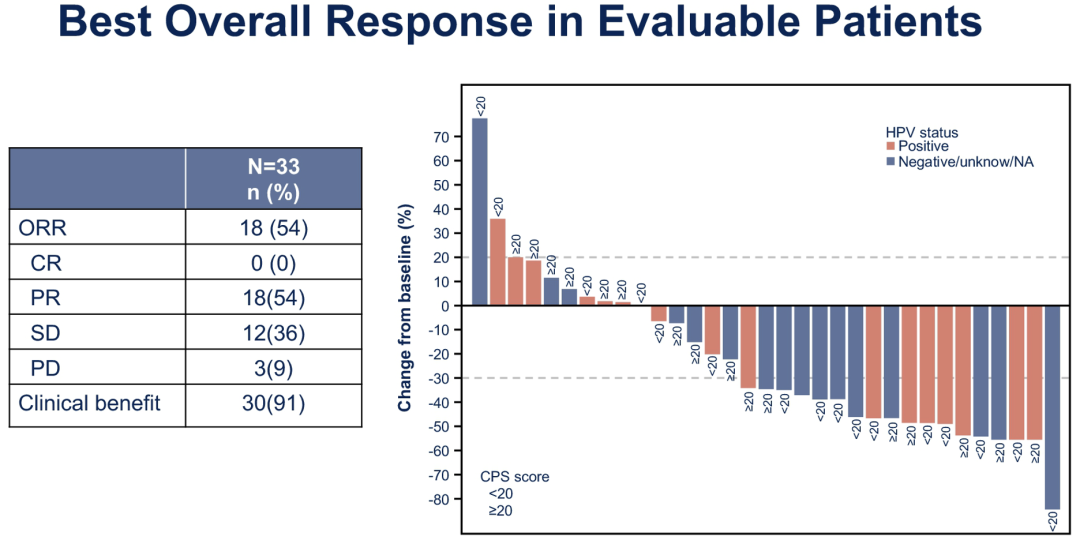
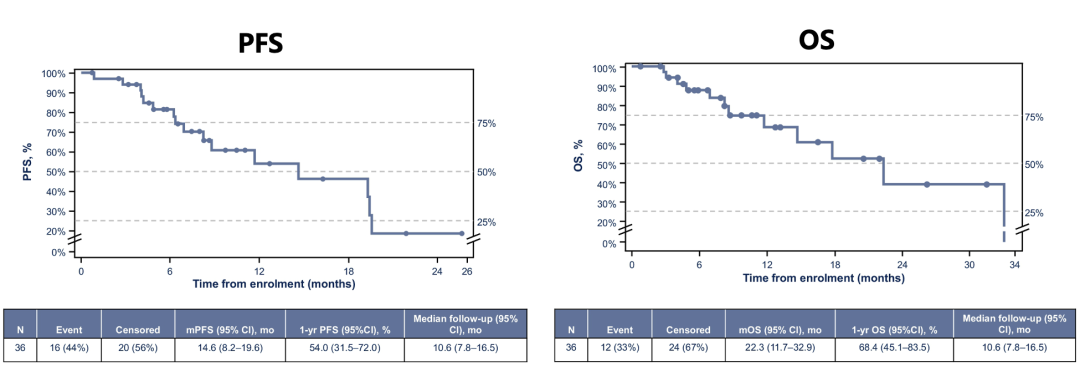
In addition, the survival data of combined treatment is also very considerable. The median follow-up time of the study was 10.6 months, and the median total survival (OS) and the mid-level non-progressive survival (PFS) of the 36 patients in the group (OS) reached 22.3 months (95 % CL: 11.7-32.99. The month) and 14.6 months (95 % CL: 8.2-19.6 months); the 1-year OS rate and 1 year PFS rate were 68.4 % and 54.0 %, respectively.
Figure 6. ASCO #6008 Study PFS, OS data
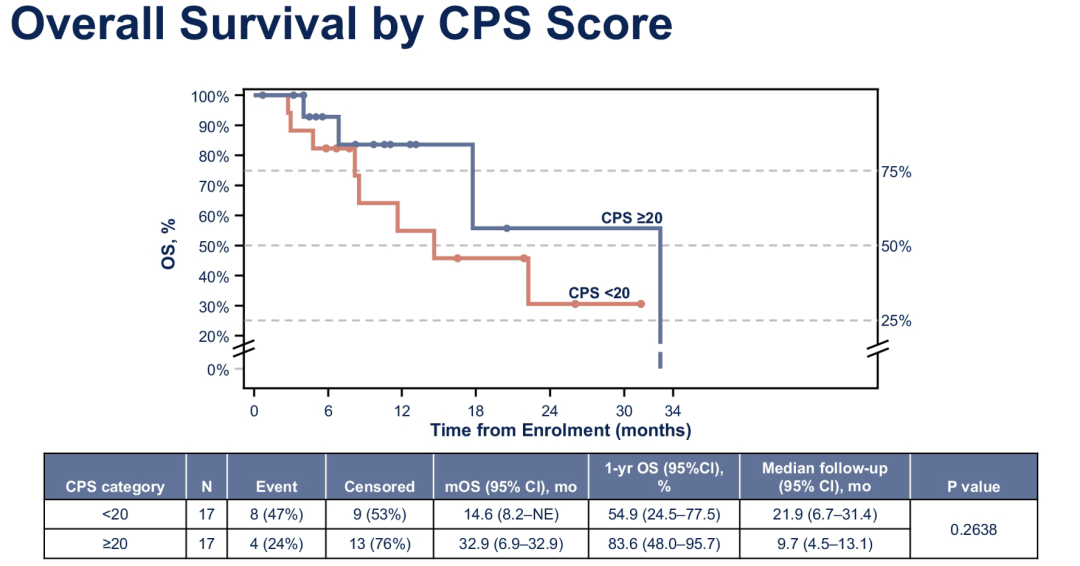
It is worth noting that the Asian group analysis shows that the benefits of PD-L1 high-expressed patients in Paborzab+Cabozantinib combined therapy are more significant, and the advantages are further locked on the basis of considerable survival data. The median OS of patients with PD-L1 CPS ≥20 is as high as 32.9 months (95 % CL: 6.9-32.9 months), and the median OS of CPS <20 patients is 14.6 months (95 % CL: 8.2-Ne month) (P = 0.2638).
Figure 7. ASCO #6008 Study data of Asian group analysis data
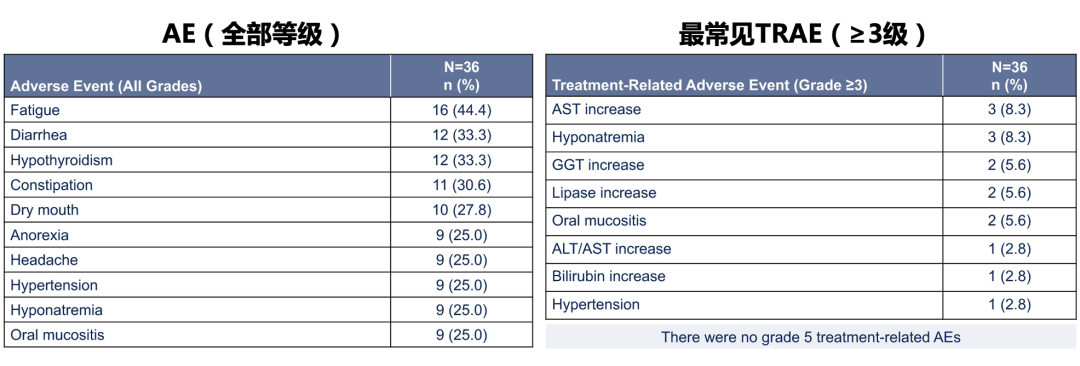
In terms of another major endpoint of research, the most common any of the most common level adverse reactions (AE) of Paborzab's combined with Cabozantinib the treatment of Cabozantinib is fatigue (44.4 %), diarrhea (33.3 %), and thyroid dysfunction (33.3 % ), The most common adverse reactions (Trams) is the increase (8.3 %), low sodium (8.3 %), and the elevated aminoly rotor enzyme (5.6 %), which are not as high as the aminotransferase (8.3 %), and did not. Level 5 treatment related adverse reactions occur. In addition, 17 patients (47.2 %) patients reduced the Cabozantinib dose to 20 mgqd due to oral mucositis, hand, foot and mouth disease, and diarrhea.
The effect of efficacy and safety, K medicine combined with the Cabozantinib plan urgently needs to enrich evidence and benefit clinical clinical
Overall, this evaluation of Paborzab combined with Cabozantinib to treat R/M HNSCC Phase II clinical studies reached the preset ORR main end point, the overall ORR was as high as 54 %, and it also obtained considerable PFS and OS data. The median OS of patients with PD-L1 CPS ≥20 is as high as 32.9 months. In terms of safety, the safety and tolerance of the combined treatment scheme is good. Paborzab is overwhelmed with Cabozantinib, and the adverse reactions are relatively controllable. In addition, the adverse reaction can be further controlled by reducing the dose of Cabozantinib, and the decrease in dose does not affect the clinical benefits of patients with R/M HNSCC.
It should be noted that there are still problems that need further exploration behind the stunning efficacy data of this study. First of all, this study belongs to phase II research. Before clinical and guiding clinical practice, its conclusion still requires phase III research to further provide evidence -based medical evidence for consolidation. In addition, I also look forward to comparing the "immune+targeted" scheme with the “immune+targeted" scheme with the basic scheme based on the basic scheme of chemotherapy, further explore the feasibility of "de -chemotherapy", and help the life of patients with R/M HNSCC patients live Increase quality.
references
[1] .mei m, et al. Theer Adv Med oncol. 2020 DEC 8; 12: 1758835920975355.
[2] .marur s, factoStiere aa .. Mayo Clin Proc. 2008 APR; 83 (4): 489-501.
[3]. China Clinical Oncology Society.
[4] .sharabi ab, et al. Lancet oncol. 2015; 16 (13): E498-509.
[5] .saba nf, et al. Cancers. 2022; 14 (5): 1202.
[6] .saba nf, et al. ASCO 2022. ABS 6008.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
Will be addicted and damaged!Do you dare to take this "smart medicine"?
In June, students in Shanghai's senior year, senior high school, and junior high s...
Published by China 丨 Holding History of Shanghai History and History of 124 people infected in the i

China Net, June 14th According to the information of the Chengdong District People...