[2022 ASCO] Revolutionary new │MHSPC field wonderful highlight express delivery
Author:Cancer Channel of the Medical Time:2022.06.15
*For medical professionals for reading reference

On June 3, 2022, the annual conference of the American Society of Clinical OnCology (ASCO) was officially held in the form of online and offline conferences. Several problems in the field of MHSPC: What is the long -term ending of the HSPC targeted transfer cooker, whether the mid -term clinical endpoint can replace the long -term OS, and what is the real world effect of the early strengthening of drugs in the early stage of drugs. Professor Zhu Yao of Affiliated Cancer Hospital analyzed and commented, and shared with readers.
2022 ascoabs 5025#
Widowed Sensitivity Sensitivity Prostatal Cancer Movedown Treatment Treatment of Target and Forecast Factors
The concept of "widow transfer" was first proposed in the mid -1990s, defined as the intermediate stage of limited diseases and extensive metastasis, and the number of metastasis was limited. A forward -looking studies have shown that Metastasis Direct Therapy (MDT) for the survival of patients is significantly related to the survival of patients with metastatic stove -targeted therapy (OMHSPC). At this conference, two summary analysis of long -term follow -up of random control tests (STOMP and Oriole) for MDT treatment for OMHSPC patients [1], at the same time evaluated the predictive ability of susceptible gene mutation characteristics to the efficacy of MDT treatment.
Study at the Stom (n = 62) and Oriole (n = 54) in the entry group, randomly receive individual MDT radiotherapy or observed OMHSPC (<3 lesions) patients. The main endpoint is PFS [defined as PSA or imaging progress time, or the time of starting androgen deprivation of therapy (ADT), or to death, calculate the first occurrence], the secondary end point is RPFS (defined as the image to the image to the image. Learning progress or death time). At the same time, this study detects the susceptible genes (including ATM, BRCA1/2, RB1, or TP53 pathogenic mutation) and MDT through the next -generation sequencing (NGS).
The median follow -up analysis shows that compared with the observation group, the median PFS of the MDT group is significantly extended. Essence In addition, the short -term observation indicators can also observe the differences between the two groups. 84%of the patients in the MDT group are reduced, while the observation group is only 41%.
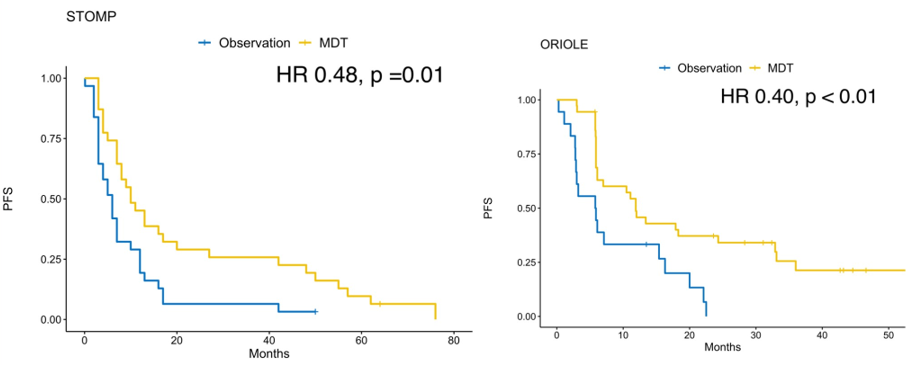
Figure 1 Stomp and Oriole Study: PFS of different treatment schemes
The incidence of prone gene mutations in the two studies is 24.3%. The median PFS of patients with negative genetic mutations is longer than the positive patients with mutation (medium 11.9 VS 5.9 months, HR 1.74, P = 0.06). The median RPFS of the susceptible gene mutation negative patients is significantly longer than the mutant -positive patients (median 22.6 vs 10.0 months, HR 2.62, P <0.01). Prosperous gene mutations can be used as a prognostic prediction factor for PFS and RPFS.
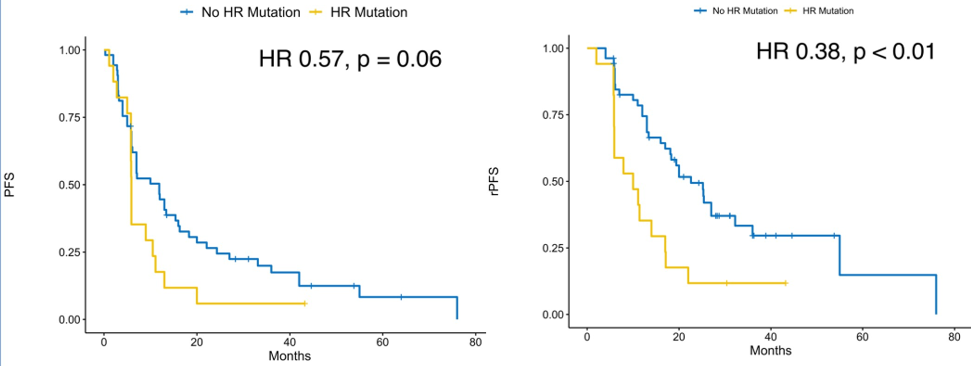
Fig
Laying analysis shows that PFS, patients with susceptible gene mutations and MDT treatment, is the longest, up to 13.4 months; and the PFS of patients with positive susrswing gene mutation but not received MDT treatment is the shortest, only 2.8 months. Studies have also found that the benefits of pervasive gene mutations positive patients from metastasis targeted therapy are relatively large (susceptible gene mutation positive group: HR 0.05, P <0.01; susceptible gene mutation negative group: HR 0.42, P = 0.01 ; Interactive inspection p = 0.12), this indicates that the susceptible gene mutation state can screen the superior group for the treatment of the metastasis.
Expert Reviews
OMHSPC is a transitional stage in the progress of prostate cancer. The domestic and foreign guidelines recommendation schemes include systematic therapy in the new endocrine drug, and local radiotherapy combined with the primary stove. In recent years, some scholars have also studied the efficacy and safety of OMHSPC received the treatment and safety of metastatic stove radiotherapy and primary stove surgery. Studies on Fudan University Cancer Hospital have shown that primary stove surgery has better RPFS and PSA-PFS than ADT single drug treatment, but further follow-up verification has long-term benefits. This article is combined to analyze the long -term follow -up results of the two metastases targeting radiotherapy research, showing that the target radiotherapy of the metastasis is better than observed and can continue to benefit. Patients with prone gene mutations are progressing faster, and they may benefit more from the targeted therapy of the metastases. It is prompted that patients with susceptible gene mutation positive OMHSPC receive more positive treatment in the early stage and benefit greater benefits.
2022 ASCO ABS 5006#, ABS 5039#
Can MHSPC's mid -term clinical endpoint "replace" the end point of traditional curative effect?
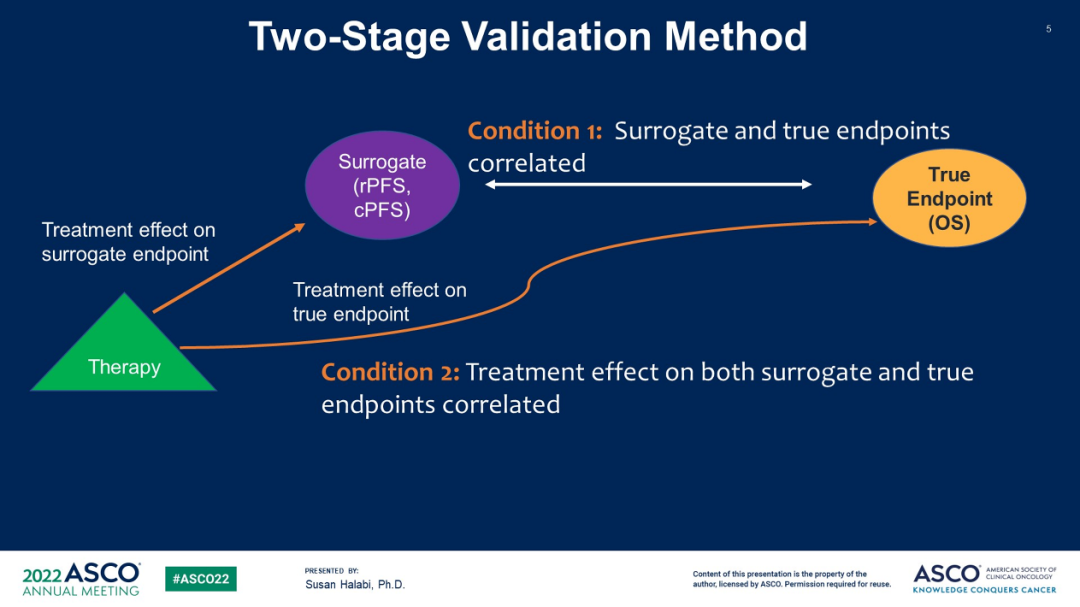
Although the total survival period (OS) is the gold standard of clinical efficacy, there are also problems such as large samples, long follow -up periods, high costs, and susceptible to other drug interference. Clinical or research is also exploring its alternative ending. The pathology is completely relieved (PCR), disease -free survival (DFS), no event survival rate (EFS), and disease control rate (DCR). The increasing development of the replacement end also brings many problems: how to choose the end point of replacement? Can the replacement end instead replace the traditional endpoint? A Stopcap M1 Collaboration Study (ABS 5006#) [2] at this conference evaluated the replacement of RPFS and CPFS as the potential mid -term clinical endpoint (ICES). RPFS is defined as the time from randomization to imaging progress (defined according to schemes) or any causes of death (prevailing first); CPFS is defined as from randomization to imaging progress, symptoms, starting new treatment or death The date of date shall prevail first. The random test of the MHSPC treatment scheme (ADT or ADT+Doxi) was selected to obtain data (IPD) of individual patients (IPD). Through the second -stage meta -analysis verification model, the alternative threshold effect (STE) was calculated. It is estimated that the survival period (OS) The minimum ICE treatment effect required for non -zero effects.
Figure 3 Research Design
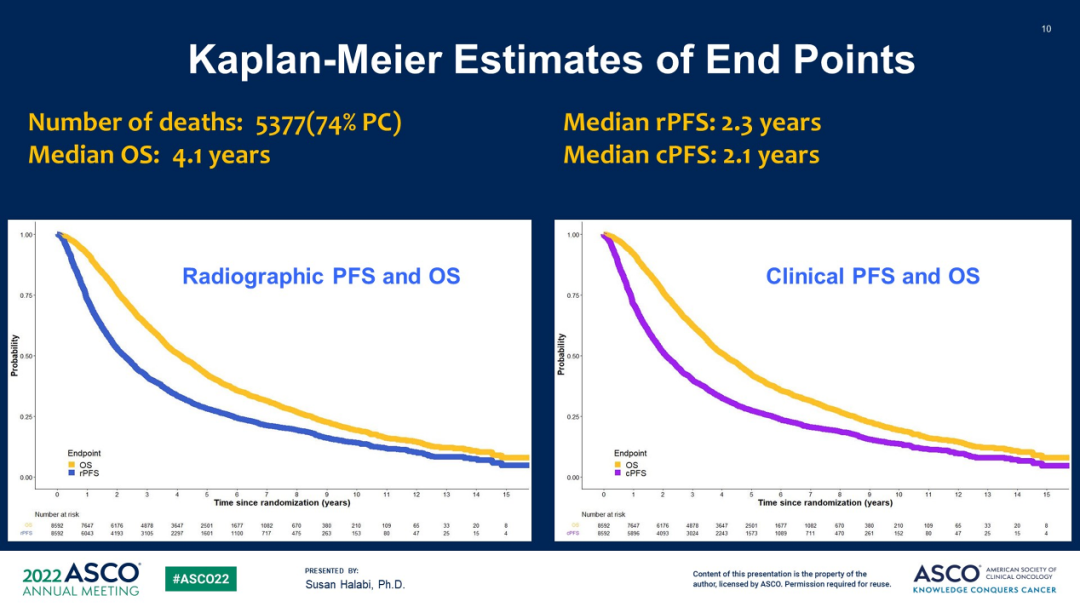
The study was included in 13 tests from 1994-2012, and IPDs involving 8,592 patients were included in stratified analysis. 5377 deaths, of which 3971 (74%) died of prostate cancer. The median age is 67 years old, the PS score of 0 accounts for 68%, and the use of ADT -based research accounts for 61%. The median follow -up time of the surviving patients is 75.6 months, and the median OS, RPFS, and CPFS are 4.1, 2.3, and 2.1 years, respectively.
Figure 4 OS, RPFS and CPFS
The results showed that the STE of RPFS was 0.83, and the STE of CPFS was 0.84.
Figure 5 The correlation between ICES and OS
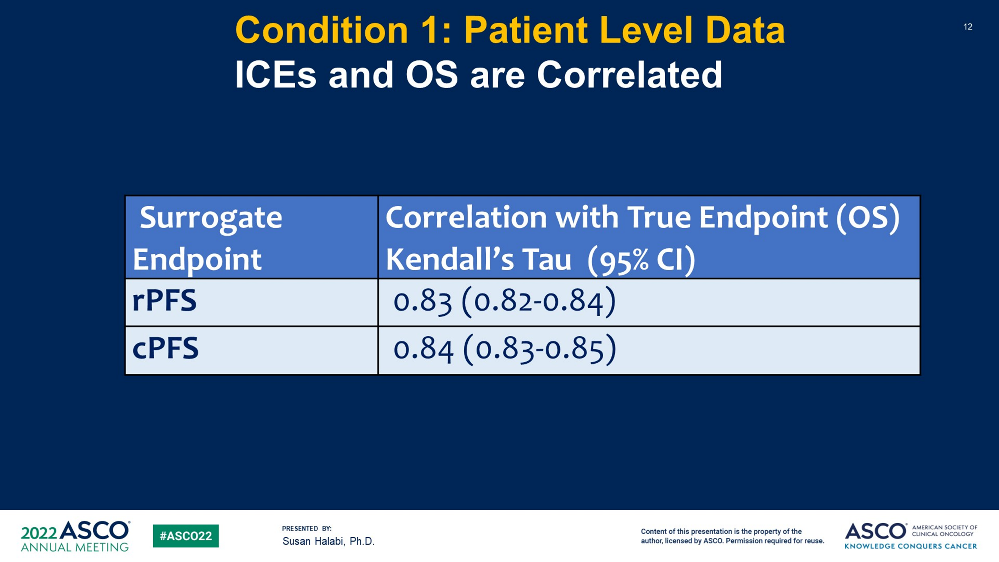
Figure 6 The correlation between RPFS or CPFS and OS
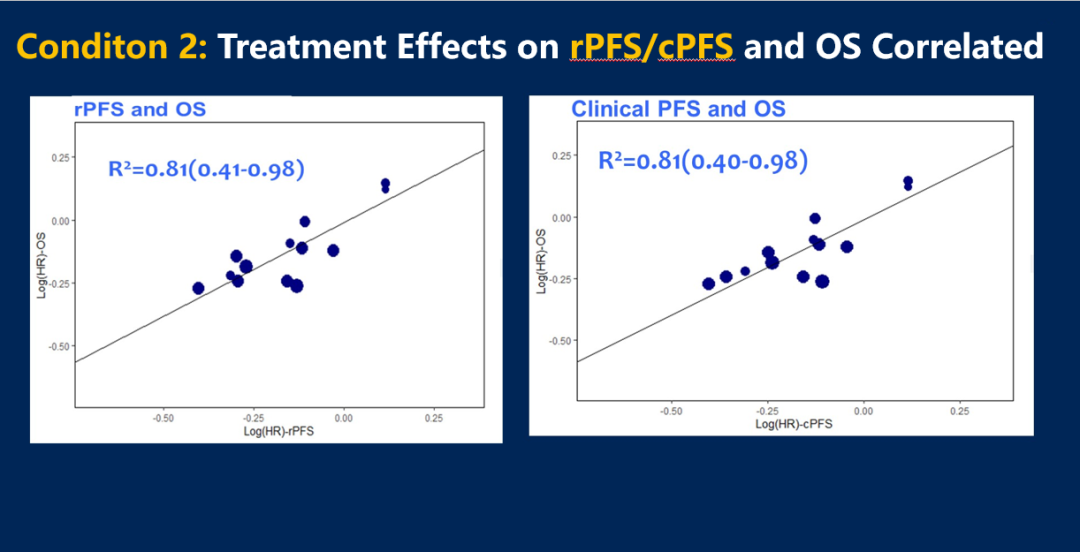
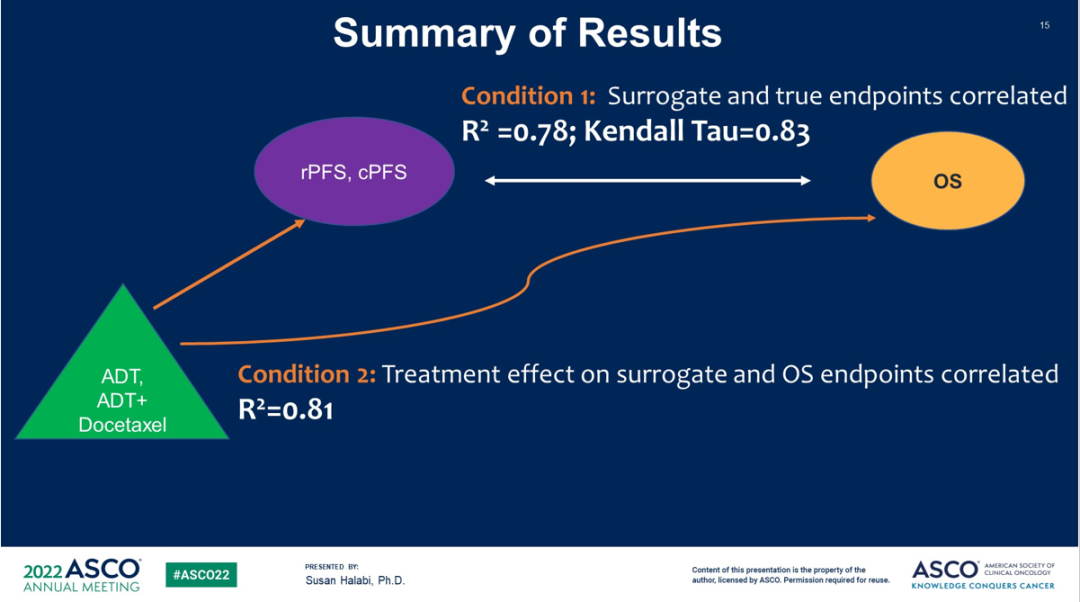
The study shows that both RPFS and CPFS can be used as an effective replacement end point for OS in the MHSPC study in Phase III.
Figure 7 Study conclusion
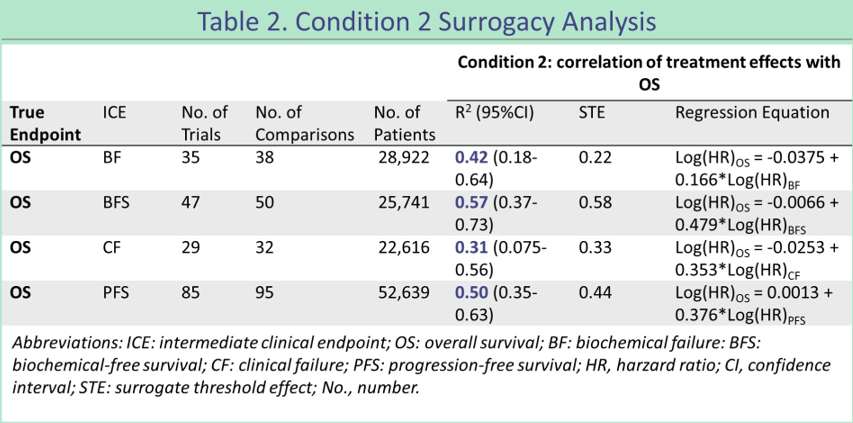
However, another summary analysis (ABS 5039) [3] concluded the opposite conclusion. This study retrieved a total of 143 (N = 75,601) report OS and ≥1 ICE [including biochemical failure (BF), clinical failure (CF), non -biochemical failure (BFS), no progressive survival (PFS) ) The clinical trial of the advanced prostate cancer of the image ± other research definitions (RPFS)] found that the end point of non -candidate meets the alternative standard. The R2 of BF (n = 28,922) is 0.42 (95%CI, 0.18-0.64), BFS (n = 25,741) is 0.57 (95%CI, 0.37-0.73), CF (n = 22,616) is 0.31 (95%CI , 0.0075-0.56), PFS (n = 52,639) is 0.50 (95%CI, 0.35-0.63), and RPFS (n = 52,548) is 0.50 (95%CI, 0.35-0.63).
Figure 8 Alternative standard analysis
BFS and PFS do not meet the alternative standards in the sub -groups of sensitivity or resistance or treatment type. When evaluating the progress of the definition of imaging (excluding or accompanied by clinical progress), the PFS of the entire group and the state of potential does not meet the alternative standard. Sensitive analysis shows that the alternative qualification of all candidates will not change over time.
The study shows that the commonly used clinical endpoints are not an effective replacement end point for OS. In the future, it is necessary to further explore the constructing compound endpoint.
Expert Reviews
The End of the Replacement as an effective indicator of the clinical ending and reducing clinical observation time. The application in the development and review procedures of prostate cancer is becoming increasingly widespread. Opinion. In the future, we respond to continuous monitoring of alternative endpoints and tap other alternative end points such as PSA depth response to better understand the correlation between the alternative end and clinical ending indicators. In addition, the real world evidence can also be used as a supplement, which is confirmed with the alternative ending index, thereby increasing the availability of drugs.
2022 ASCO ABS E17052#
Thinking: The impact of drug development progress on the prognosis of metastatic prostate cancer survival
In the past ten years, with the new androgen receptor pathogenic inhibitors [Aibira Dragon (2011), Enzalu (2012), Apa Taamine (2018), and Radium 223 (2013)] The approval of therapy, the survival of patients with metastatic prostate cancer (MPCA) has been continuously improved. However, the survival benefits at the level of crowd are unclear. A retrospective analysis released by this meeting [4] aims to evaluate the impact of drug research and development progress in the past ten years on the survival of the new diagnosis of MPCA patients.
Studies were reported by retrieval of the SEER database and included 33,969 new MPCA patients from 2000 to 2018. According to the diagnosis, patients are divided into two treatment periods: 2000-2010 [period A, 15,433 (45.4%)] and 2011-2018 [period B, 18,536 (54.6%)]. Use Kaplan-Meier to assess prostate cancer-specific survival (PCS) and overall survival (OS); PCS and OS between the two periods of natal tests. Single variables and multi -variable COX regression models are performed to determine the significant collaborative variables of PCS and OS. The results showed that there were no significant differences in clinical pathology and socio -economic characteristics of the two periods. Compared with the period A, the median PCS (36 VS 30 months, P <0.001) and OS (30 VS 25 months, P <0.001) are longer. In PCS's multi-variable models, the diagnosis of period B is significantly reduced to the risk of prostate cancer death (HR: 0.89, CI: 0.85-0.94, P <0.001). Age & 80 (HR: 1.6, P <0.001), PSA & 50 NG/ML (HR: 1.3, P <0.001), and low -differentiated histological (HR: 2.0, P <0.001) are important predictive factors for PCS deterioration. Spain (HR: 0.94, P = 0.02) and the median income of household income & 75,000 US dollars (HR: 0.86, P = 0.007) are related to better PCS. In the OS multi-variable model, the diagnosis of the period B is significantly related to the risk of death (HR: 0.89, CI: 0.85-0.93, P <0.001). Age & 65 (HR: 1.26, P <0.001), PSA & 50 NG/ML (HR: 1.30, P <0.001), and low -differentiated tissue (HR: 1.6, P <0.001) are related to less OS. Spain (HR: 0.94, P = 0.01) and home income median & 75,000 US dollars (HR: 0.81, P <0.001) are related to better OS.
In summary, the new diagnosis of MPCA phase B (2011-2018) is related to better PCS and OS improvement. However, the risk of death observed in this crowd-based study was only reduced by 11%, which was lower than 20-38%of clinical trial reports.
Expert Reviews
With the listing of innovative drugs, the early intensive treatment of new endocrine drugs has significantly improved the overall survival benefits of MPCA patients. This retrospective analysis corresponds to the long -term follow -up data of the Abbit Dragon group in StamepeDe's study before. After 73 months after medium follow -up, the ADT Dragon combined with the ADT group has significantly reduced the risk of death compared with the ADT group, and increased the 5 -year survival rate to a 5 -year survival rate to 60%, indicating that the treatment of new endocrine drugs is moved forward, that is, when diagnosis of MHSPC, new endocrine therapy is used. Compared with ADT, the patient's 5 -year survival rate has been greatly improved. However, in terms of reducing the risk of death, this study is largely different from previous clinical trials, which may be related to various factors such as innovative drugs, diagnosis and treatment of medical institutions, and patient economic conditions.
Expert Introduction
Professor Zhu Yao
Chief physician, associate professor, doctoral supervisor

Deputy Director of Urology Department
Secretary -General of the Prostate Cancer Committee of the China Clinical Oncology Society
Vice Chairman of the Youth Council of the Shanghai Anti -Cancer Association
Deputy Leader of the Precision Team of the China Anti -Cancer Association Urinary Men's Reproductive Cancer Special Committee
The May 4th Medal of the Youth of Fudan University in 2019
May 4th Medal of Youth, Shanghai Health and Health Committee in 2019
2018 Outstanding Youth Medical Talent of Shanghai "Medical Garden"
The 7th Fudan University Top Ten Medical Youths
The third batch of Fudan University Zhuoxue Talent Program
Selected in 2015 Shanghai Youth Science and Technology Qi Mingxing Program
He is the editorial board of the PROSTATE CANCER and PRostatic Disease
Published 8 papers with communication authors in EUROPEAN UROLOGY and Journal of Urology to win the first prize of the Shanghai Science and Technology Progress Award for the second completion
National Natural Science Foundation review expert
references:
[1] .deek mp et al. 2022 ASCO ABS 5025.
[2] .halabi s et al. 2022 ASCO ABS 5006.
[3] .Gharzai la et al. 2022 asco ABS 5039.
[4] .adekolujo OS et al. 2022 ASCO ABS E17052.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
Tai'an City Pharmaceutical and Medical Device Industry Chain Class and Danish Embassy in China to carry out video investment investment docking meetings

In order to accelerate the development of the pharmaceutical and medical device in...
[Epidemic Speed Report] From 0:00 on June 16th to 24:00, there are no new local diagnosis cases in Yunnan
At 0-24 on June 16, there were no new confirmed cases in the province.There are 7 confirmed cases in the existing overseas input.A total of 1,525 confirmed cases were cured, and 1,518 cases were cured