[Transfer] Patients may not be enough, blood tumor CAR-T enters the era of overcapacity
Author:Yaizhi.com Time:2022.07.29
[Transfer] Patients may not be enough, blood tumor CAR-T enters the era of overcapacity
Source: amino observation/Fang Taozhi
From "Shen medicine" to "leftovers", CAR-T only took five years.
In 2017, the first "magic medicine" CAR-T was approved to go public, opening the prelude to the era of cell therapy. Later, the track gradually heated up, and global pharmaceutical companies flocked.
Today, after 5 years, although CAR-T therapy has been approved less than 10 products, after the research, the army is sufficient and it seems to enter the era of overcapacity.
According to GLOBALDATA data, more than 800 cell therapy products against hemoma are currently in the clinical stage, most of which are CAR-T therapy. The indications developed and developed by these CAR-T therapy are mainly concentrated on five types of hematoma.
In view of this, the scene of PD-1 with a bucket may not focus on CAR-T therapy. The product is enough, but the patient is not necessarily enough. Some pharmaceutical companies may be injured.
/ 01 /
Hundreds of CAR-T, compete for five hematoma indications
In 2017, the US FDA approved two CAR-T listing, and the era of CAR-T kicked off. For a while, this kind of cell therapy expected to cure cancer has become popular, and many domestic and foreign pharmaceutical companies have entered the game.
So far, eight CAR-T therapy has been approved worldwide. Among them, 6 CAR-T in the United States has been approved by the FDA, and there are two domestic models.
The reason why many pharmaceutical companies are willing to enter the game are for business prospects. Because of the advantages of efficacy, the future prospects of CAR-T therapy may not be too bad.
GlobalData predicts that the global tumor cell therapy market is expected to exceed $ 37 billion in 2028. Although this goal may not be achieved. The ideal and reality will always have a gap that is difficult to cross.
At present, several CAR-T therapy can only be said to be mediocre. Take sales data in 2021 as an example. Five CAR-T sales listed in the United States in 2021 were only $ 1.709 billion. It is far lower than PD-1, BTK, ADC and other popular tracks, which can only be ranked ninth in the world.
Among them, the best sales are Geely's Yescarta. For four years, Yescarta's sales in 2021 were US $ 695 million. In the case of total sales less than market expectations, the sales growth rate has slowed down.
Recalling that year, Geely made a cost of $ 11.9 billion to acquire Kite to get Yescarta. Now it may be false.
The commercialization of global CAR-T therapy is lower than expected, which is limited by many factors, including the market that needs to be cultivated. But even if the market size can reach GlobalData expectations in the future, it is still difficult to support the future of all pharmaceutical companies.
According to GLOBALDATA data, more than 800 cell therapy for hemoma is in the clinical stage, most of which are CAR-T therapy.
In addition, the indications of these CAR-T therapy are quite concentrated. Essence
Faced with the R & D trend of CAR-T therapy, I believe that many people will have the same question. Do we really need so many homogenization CAR-T therapy in blood tumors? The answer is obviously no.
/ 02 /
Patients may not be enough, not all CAR-T therapy has the future
Although many pharmaceutical companies are developing hot now, they must face a question in the future: Is patients sufficient enough?
Taking the most intense acute lymphocytic leukemia indications in global competition as an example. As of now, more than 250 CAR-T therapy has been carried out for this indication.
Acute lymphocyte leukemia is only a rare disease, and the patient group is not large. Take American data as an example. According to the prediction of the American Cancer Association, about 6,660 patients with acute lymphocyte leukemia in the United States in 2022.
There are fewer new patients, and the size of the group of patients must be limited. In addition, not all patients are suitable for CAR-T therapy, because the scale of potential patients will appear more forced.
Based on this perspective, the competition of this indication must be the speed of life and death. The subsequent ME too products may not even drink the soup.
You may say that most pharmaceutical companies have developed this indications that the purpose may not be declared to be listed, but just an attempt to verify whether it is feasible for CAR-T therapy. After all, CAR-T therapy has been proven to be effective in acute lymphocyte leukemia.
In other words, this is just the stepping stalls of many pharmaceutical companies, and the future battlefield is in other markets. But the scale of hundreds of competitors is destined to be difficult to lie flat.
In the era of overcapacity of hematoma CAR-T therapy, it may occur not only overseas, but also in China.
According to data from BOC Securities, as of May 2022, more than 20 domestic companies have participated in the competition of CAR-T therapy, and most of them are targeting the CD19 target of hematoma.
For some ME TOO drugs with slower progress, the future prospects may gradually dim.
/ 03 /
The roll of rolls, the fruit of picking high, and the cost of low product, so is there still a future for CAR-T therapy? The answer must be yes.
Although hematoma indications have excess capacity, solid tumors still need to be overcome. At present, CAR-T therapy for solid tumors has not been approved for listing.
The reason is that there are too many levels that overcome solid tumors. For example, in the field of physical tumors, it is difficult for CAR-T to find the goal of "precise blows" due to a special tumor micro environment. Essence
But risk and opportunities coexist. Once CAR-T can break the barriers of physical tumors, then it can break the fate of the inner roll in blood tumors and have a broader market.
The scale of patients with solid tumors is much larger than hematoma. In 2019, there were 18.5 million global cancer patients, of which 17.3 million were solid tumors, and 93%of patients with physical tumor.
More importantly, most of the lack of effective treatment methods of most physical tumors, or the survival benefits brought by existing therapy are still effective. Therefore, if CAR-T can overcome the future of solid tumors, it is not limited.
At present, many pharmaceutical companies are challenging the difficult bone of physical tumors. Domestic aspects, Koji Pharmaceutical targeted CAR-T of CLDN18.2 protein, and is undergoing clinical trials for treating cancer/esophageal and gastric adenocarcinoma and pancreatic cancer; The indication of the tumor is also in progress. In addition, legendary biology, Chongqing precision biological and other pharmaceutical companies are eager to try.
In addition to the high fruit of picking physical tumors, another path of CAR-T breaks the inner roll is to upgrade the product and reduce the cost of CAR-T therapy.
At present, the pricing of eight CAR-T therapy that has been listed around the world has exceeded one million. The cost of treating a house to catch up with a house in a third-tier city is naturally difficult to make CAR-T fly into the home of ordinary people.
Therefore, if you want to increase the penetration rate of CAR-T, reducing costs is a feasible idea.
The currently available CAR-T therapy is a highly personalized customized product, and it is difficult to produce a large-scale effect and cause the cost. If you can upgrade the highly customized CAR-T therapy to the assembly line products for mass production, it will undoubtedly have more advantages.
Based on this, the general-purpose CAR-T therapy came into being. Many domestic pharmaceutical companies have begun to challenge the research and development of spot CAR-T therapy.
In December 2020, the GC007G injection of GC007G developed by the Jixi Bio was approved by clinical clinic, becoming the first universal CAR-T therapy that entered the clinical stage in China, and is currently in the second phase of the clinic.
On March 17 this year, the CTA101 independently developed by Beiheng Bio has officially obtained a clinical trial implicit permission, becoming the second universal CAR-T therapy in China to enter the clinical stage.
In addition, there are many pharmaceutical companies such as legendary creatures, Koji Biological, Breastsens, and Senlang Bio who have deployed spot CAR-T.
Of course, the reason for challenges is not small. How to balance safety and efficacy is a problem faced by every pharmaceutical company.
But anyway, as long as the pharmaceutical companies are willing to work hard, the hope of CAR-T therapy to break through. Pharmaceutical companies that are so -called challenges and opportunities coexisting and being the first to make breakthroughs will also be able to win a lot of awards.
Disclaimer: This article is the content of Yaozhi.com, and the copyright of the pictures and text belongs to the original author. The purpose of reprinting is to pass more information, which does not represent the viewpoint of this platform. If the content, copyright and other issues are involved in the work, please leave a message on this platform, and we will delete it as soon as possible.
- END -
Sleeping in a summer mat, what is the itching allergy?
Many friends have the habit of sleeping mats in summer, but if the mats do not do ...
Changde First Traditional Chinese Medicine Hospital completed the first province's first outpatient clinic slow special disease costs to settle directly
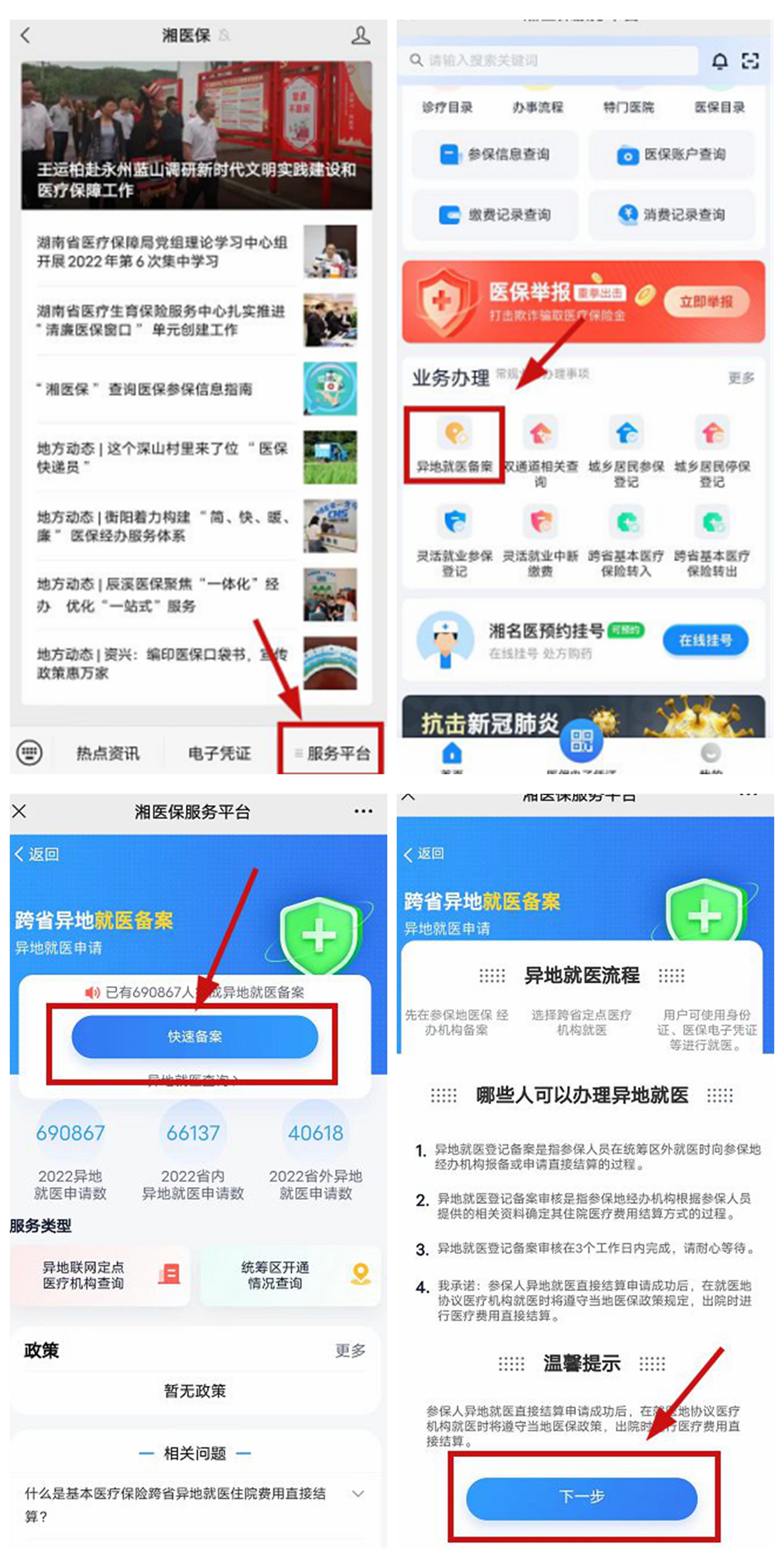
Huasheng Online News (Correspondent Wu Xiuying) In order to better meet the needs ...