The IPO of pharmaceutical companies has new regulations; the state has a psychiatric medical center;
Author:Kenji Bureau Time:2022.07.31
This week, the domestic new crown epidemic has been fluctuating. According to the latest report by the National Health and Health Commission, on July 30, there were 116 newly confirmed cases nationwide, and there were currently 1,731 confirmed cases in the country.
At the national level, the State Council ’s joint prevention and control mechanism requires that the test results of the new crown nucleic acid testing are mutually recognized nationwide. Beijing has stipulated that the purchase of "four types of medicines" such as anti -fever and cough, as long as nucleic acids are performed within 72 hours, they will no longer be "pop -ups". Since July 30, Jiangxi has not performed yellow code management within 48 hours.
In terms of market, in the first half of this year, the number of IPOs in pharmaceutical companies was only 21, a decrease of 8 compared with the same period last year. This week, the CSRC officially stipulated the content and format of the prospectus of pharmaceutical and medical device companies, and the market management of pharmaceutical companies will be more stringent in the future.
Domestic new crown medicines were tired. The real creature "Azfdin" was approved to be listed and became the first domestic new crown oral medication; Tengshengbo Pharmaceutical announced that the company's new crown and antibody against Omikon BA.4/5 and BA.2.12.1 subtypes Mutant strains remain neutral.
More information is sorted as follows:
Heavy policy
1. The National Health and Health Commission sets up the National Psychiatric Medical Center
On July 29, the National Health and Health Commission issued an announcement that according to the relevant arrangements of the National Medical Center and the National Regional Medical Center during the "Fourteenth Five -Year Plan" period, it will be in Beijing, Shanghai, and Hunan Province. , Shanghai Mental Health Center, Xiangya Second Hospital of Central South University set up the National Mental Disease Medical Center for the main body.
The announcement also stated that in addition to conducting business guidance on the work of the medical center in the future, the National Health and Health Commission will also issue special tasks and supporting funds to carry out performance evaluation and supervision of the implementation of the work tasks of the medical center each year.
2. The two ministries and commissions jointly issued a document to strengthen the supervision of medication for medication
On July 27, the State Health and Health Commission and the State Administration of Medicine jointly issued the "Notice on Further Strengthening the Management of Medication Safety Management and Improvement of Reasonable Medication". Requirements.
The "Notice" emphasizes that it is necessary to strengthen the management of the use of rational drugs, anti -microbial drugs, anti -tumor drugs, proton pump inhibitors, and traditional Chinese medicine injections. In addition, when selecting children's medication, it may not be limited by the "one product, two regulations" and the number of drugs for the total drugs, and increase the scope of medication.
3. Shenzhen's highest subsidy 50 million yuan to support pharmaceutical companies
On July 26, the Shenzhen Municipal Development and Reform Commission officially issued three policies and measures such as the "Shenzhen Municipality to Promote the High -quality Development of the Biomedical Industry Cluster" and support Shenzhen's bigger and stronger biomedical industries.
The "Measures" mentioned in particular that it is necessary to speed up the construction of major industrial public service platforms such as CRO, CDMO, GLP, Pharmaceutical Discovery Platform, and MAH Comprehensive Service Platform, and funded at 40%of the total investment of the project, with a maximum of not more than 50 million yuan. In addition, major industrial projects that are related to the core technologies such as "card neck" such as new target chemicals, antibody drugs, and gene drugs will be funded in accordance with 40%of the total investment in the project, and the maximum is not more than 300 million yuan.
Industry affairs
1. Wu Zunyou: Monkey acne will not be popular in China
On July 24, after Taiwan reported the first diagnosis case of monkey acne, Wu Zunyou, chief expert of the China Centers for Popular Diseases, posted on Weibo on the 25th that from the perspective of the spread of monkey acne in Europe and the United States, the transmission of monkey acne transmission It is only a matter of time to enter the mainland and Hong Kong and Macao regions. Due to the limitation of communication methods, the speed of acne spread and the scope of crowds will not be as popular as the new crown, and the epidemic of monkey acne will not cause large -scale popularity in my country.
In addition, Wu Zunyou said that the earliest attack on the epidemic of monkey acne is likely to be a male behavior group, and then spread to other people. In terms of prevention, one is to avoid contact with the infected animals, and the other is that people who are not infected as much as possible should be directly contacted.
2. The request for new IPO disclosure of the CSRC is more stricter
On July 29, the Securities and Futures Commission officially released the "Guidelines and Format Guidelines for the Prospectus of the Company Prospectus in the Drug and Medical Device Business". From the aspects of the prospectus risk factors, business and technology, etc. The content and format are made.
It is worth mentioning that in terms of product disclosure, the "Guidelines" requires companies to announce "comparison of safety, effectiveness, and availability." In addition, the "Guidelines" also stipulates that enterprises need to disclose the product "patent rights due to the expiration of patent rights or a significant decline in operating income or net profit."
3. Alzheimer's severe dissertation is suspected of fraud
On July 21, "Science" published an investigation saying that many papers in the field of Alzheimer (AD) and neurosciences of the University of Minnesota, a neuroscienceist, were suspected of academic fraud, of which Including a foundation related to the AD pathogenesis "β amyloid protein hypothesis". "Science" said that this paper "misleading the study of Alzheimer's disease in the world for 16 years."
Academic fraud is common. The reason why the investigation of "Science" has attracted widespread attention, which may be related to the repeated defeats of drug development in recent years and continuous controversy: abroad, Bo Jian's "first approved AD drug after 13 years" Aduhelm has been strongly resisted by strong resistance In China, the "971" of Green Valley is also frequently questioned.
However, according to the "China News Weekly" report, most of the neuros scientists are said that even if these papers are finally proven to be fraud, the impact on academia and industry is "not as serious as the outside world thinks." 4. Two major synthetic biology companies merge
On July 25, Ginkgo and Zymergen, two synthetic biology companies in the United States, announced that the two parties had reached an agreement. Ginkgo would acquire Zymergen in full -stock transaction. The acquisition case was worth about $ 300 million.
Both Ginkgo and Zymergen were established around 2013 and together with Amyris, which develops biofuels and became "three giants of synthetic biology." Both Ginkgo and Zymergen are positioned as a construction platform company to create a biological manufacturing platform that uses machine learning and other means. However, Ginkgo focuses on platform construction, and Zymergen attaches importance to product development. These two models also basically represent the current research and development path of synthetic biology companies.
New drug inventory
1. The world's first direct injection of the brain AAV gene therapy is listed
On July 21, PTC Therapeutics, a listed company in Nasdaq, announced that the European Commission approved genetic therapy UPSTAZA to be listed to treat aromatic L-amino acid decarcar deficiency (AADCD), becoming the world's first first injection of the brain to get the brain. Batch genetic therapy.
UPSTAZA is a disposable Ji drink. The first test patient was treated in 2010. Data published by PTC Therapeutics show that after three months of receiving UPSTAZA treatment, patients have been effectively improved, and the curative effect continues until 10 years after receiving treatment. In addition, the cognitive ability of all patients who receive treatment have been improved.
2. The only monkey acne vaccine in the world is listed in Europe
On July 25, Danish Biomedical Bavarian Nordic announced that the European Commission has approved the company's MVANEX to be listed, and all EU member states, as well as Iceland, Liechtenstein and Norway.
Imvanex was developed by Bavarian Nordic and the US government. It was originally a non -reproducible ceiling vaccine, but animal data showed that it was effective in preventing monkey acne. At present, this vaccine has been approved in the United States and Canada to prevent monkey acne.
3. Ideaya announced the clinical data of new cancer therapy
On July 28, Ideaya Biosciences announced that the small molecular MAT2A inhibitor Ide397 developed based on the principle of "synthesis and death" was obtained in Phase/II clinical trials: 75%of patients who received high doses of treatment, 75%of patients who received high doses of treatment Circular tumor levels have decreased significantly.
"Synthetic death" is a more popular anti -cancer drug development concept in recent years. In principle, cancer cells carry a large number of genetic mutations. These mutations make them have the characteristics that they do not have healthy cells, but they may also produce unique weaknesses. By studying these weaknesses to kill cancer cells, at the same time avoid health cells from being injured by harm Essence
### ## Psychiatric Medicine Center#

- END -
Continuous unilateral tinnitus, you must be alert to listening to neuroma
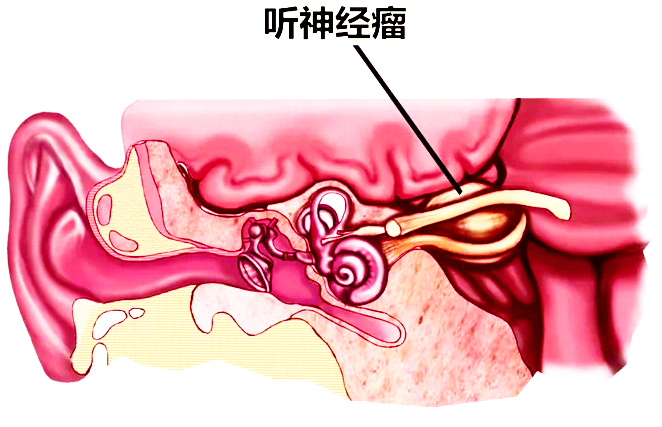
The ears occasionally buzzed or hearing decreased, accompanied by discomfort such ...
Lanzhou Chengguan District is urgently traced, involving these places ...

Announcement of Prevention and Control of Xinguan Pneumonia in Chengguan District,...