State Drug Administration: Strengthen the monitoring of information on the network transaction information on the platform
Author:Zhongxin Jingwei Time:2022.06.16
Zhongxin Jingwei, June 16th. According to the website of the State Drug Administration, on June 14, the State Drug Administration held a chamber of commerce by the medical device network transaction management risk. It is mentioned that the frequency and intensity of the monitoring of the network transaction information of the medical device in the platform.
At the meeting, the Southern Medical Economics Research Institute of the State Drug Administration notified the recent medical device network transaction monitoring situation and the disposal of the clues of illegal and violations of the online transactions; The person in charge reported on the implementation of the main responsibility of the platform, the special rectification requirements of the State Drug Administration's drug safety, strengthened the management of network sales enterprises in the platform medical device, and standardized the online sales behavior of medical device online; the Beijing Pharmaceutical Regulatory Bureau, Shanghai Pharmaceuticals, Shanghai Pharmaceutical Bureau Relevant responsible comrades of the Supervision Bureau and Zhejiang Provincial Pharmaceutical Supervision Bureau reported on strengthening the supervision of medical device network transactions, strengthening dynamic monitoring of medical device network transactions, severely cracking down on illegal behaviors of medical device network transactions, improving the ability to prevent and control of network transactions in medical device, strengthen grassroots levels at the grassroots level The construction of supervision teams.
The meeting pointed out that in recent years, the State Drug Administration has continuously improved the construction of the regulatory regulations and regulations of medical device network transactions, deploying the local drug supervision departments to conduct in -depth medical device "Qingwang" operations and medical equipment quality and safety risks to investigate, and increase medical device network transactions Monitoring and illegal and illegal acts have continued to consolidate the main responsibility of the third -party platform of medical device network transaction services, and achieved certain results. Recently, the medical device network transaction monitoring has found that individual enterprises that settled in the platform have violations of laws and regulations. Relevant e -commerce platforms should attach great importance to it, carefully compare the requirements of laws, regulations and regulations, and carry out comprehensive self -investigation and rectification of e -commerce platforms to perform legal obligations.
The meeting requested that the third -party platform of medical device network transaction services strictly implements the main responsibility of the platform, ensure the quality and safety of the online sales of medical device networks, strengthen internal management and personnel training, and ensure continuous compliance of online transaction services. At the same time, strict qualification review should be strictly entered. Carefully checking the business license, registration certificate, and filing voucher information of the enterprise medical device business must be comprehensive, accurate, complete, and meticulous. If necessary, consult the issuance department and verify it. "Outside the door."
In addition, dynamic monitoring should be increased to deal with reports in a timely manner. At the same time, we must continuously strengthen monitoring forces, innovate monitoring methods, strengthen the frequency and intensity of monitoring information on network transaction information in the platform, and discover violations of the "Regulations on the Supervision and Administration of Medical Device" and "Measures for the Supervision and Administration of Medical Device Network Sales Supervision and Management" shall shall Immediately take measures such as stopping network transaction services, and report relevant clues to the drug regulatory authorities in a timely manner for suspected illegal entry enterprises, and assist in providing relevant evidence materials to work together to deal with illegal investigation and disposal.
The third -party platform of the participating medical device network transaction services stated that it will further implement the main responsibility of the platform, continuously optimize the qualification audit system, increase the investment of professional audit personnel, improve the frequency of artificial inspections and the effectiveness of the audit, and standardize the online transaction market order in the platform. Resolutely crack down on medical device network transactions and violations of laws and regulations, and effectively ensure the safety of consumers to buy weapon. (Zhongxin Jingwei APP)
Pay attention to the official WeChat public account of JWVIEW (JWVIEW) to get more elite financial information.
- END -
The vaccine arrives one after another!It's about influenza, Zhangzhou's latest answer
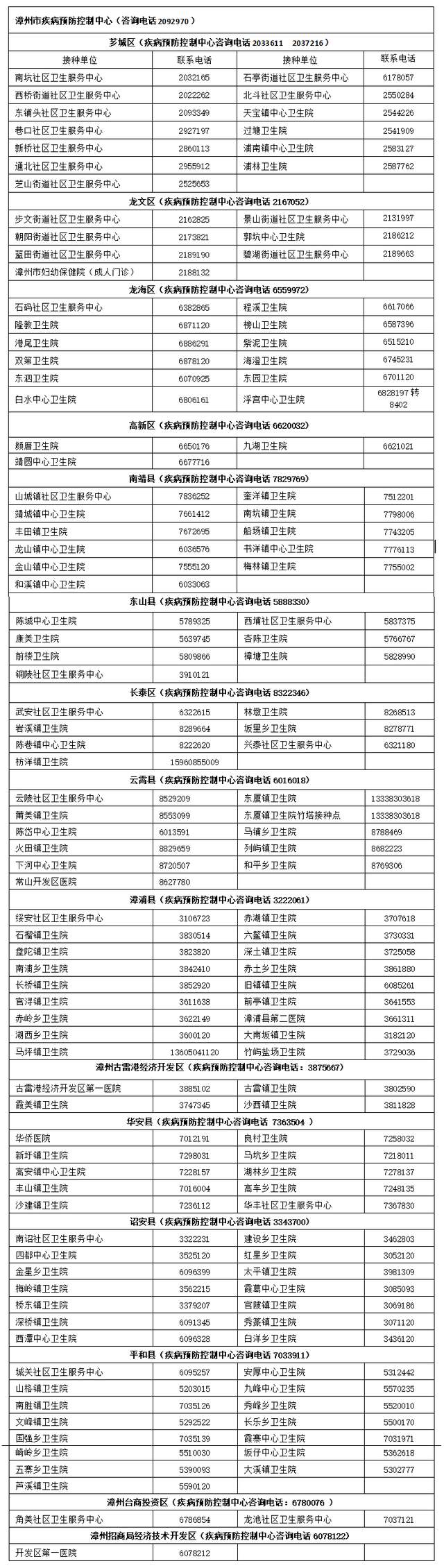
Recently, the city's influenza is highPatients in some hospitals have increased si...
Netizens competed to learn to be "Ye Wen Squat" experts remind: When squatting, the knee joint will bear 4-6 times the pressure of 4-6 times and practice and "treasure" knees

Liu Genghong's Xunzi exercise was in the ascendant, and netizens fascinated Ye Wen...