The first stock of domestic new crown oral medication?Real biological biological Delivery Tables have no income loss to increase losses
Author:First wind Time:2022.08.05
On August 4, Real Biotechnology Co., Ltd. submitted a prospectus to the Hong Kong Stock Exchange. If the successful listing will become the first domestic new crown oral medication.
In November 2021, the real creature completed a round B financing of 560 million yuan, with a pre -investment valuation of 3 billion yuan and a post -investment valuation of 3.56 billion yuan.
The company's core product Azf was designated as an innovative medicine with broad-spectrum antiviral activity. He was approved for the treatment of HIV infection and COVID-19 in July 2021 and July 2022, respectively. The first Chinese company developed oral antiviral drugs approved by the State Drug Administration to treat COVID-19.
The real creature said that with its own production capacity, the annual production capacity is about one billion tablets Azfdin, and it has fully prepared to start Azfdin's commercial sales.
According to the prospectus, as RNA dependent RNA polymerase (RDRP) inhibitors, Azf must effectively inhibit the copy of the SARS-COV-2 of COVID-19. In addition, its potential is still effective for neutralized antibodies and vaccines. The new variant of the escape virus is still effective. This is because according to the research on the new variants, the target of neutralized antibodies (virus spiny protein) has Specific mutations, while Azfdin's target (virus RDRP) is relatively conservative and the mutation rate is low.
The following table is the stage of product portfolio and drugs or candidate drugs of real creatures:
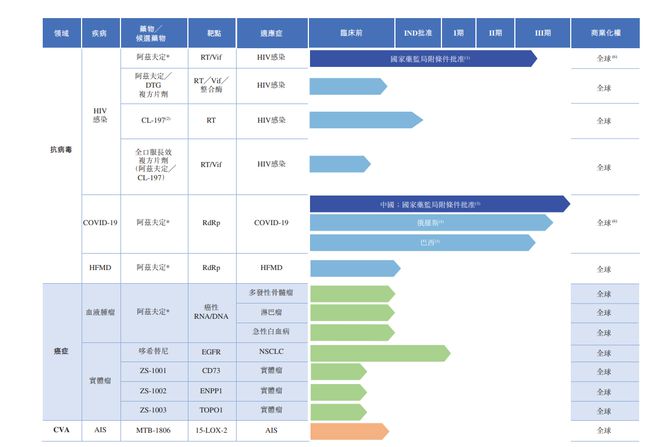
The real biological research and development pipeline involves innovative drugs that treat viral, tumor and cerebrovascular disease, including:
CL-197, a oral long-acting purine nucleoside antiviral drug for HIV;
Dokinib, a third representative of the skin growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) anti-tumor candidate drug, compared with the widely used third-generation EGFR-TKI, its potential has become safer Drugs with lower poison and side effects;
And MTB-1806, a small molecular candidate drug for acute ischemic stroke. By observing in preclinical research, its lower dose-level administration scheme can reach a pupar (a national drug supervisor (a national drug supervision supervisor (a national drug supervision supervisor AIS drugs approved by the bureau) are quite effective.
The competition of innovative drugs has not yet generated income innovative drugs
The prospectus admits that the competition for innovative drug development and commercialization is fierce. Although the company considers its innovation platform to provide a competitive advantage, it is facing competition from global and Chinese pharmaceutical and biotechnology companies.
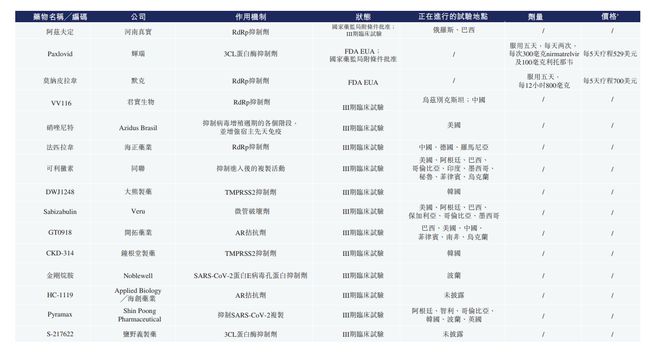
The following table contains Azfdin and other listed COVID-19 oral drugs. As of the last actual dates, the candidate drugs that may compete or jointly use with Azfdin in all countries in the world:
At the same time, the company has no income. In 2020, 2021 and as of the end of May 2022, the research and development expenses of real creatures were about 106 million yuan, 64.045 million yuan, and 114.2 billion yuan, respectively. During the same period, the losses during the period were approximately 150.9 million yuan, 197.2 million yuan, and 218.1 million yuan. Essence
In addition, the exclusive sponsor of real creatures is CICC.

- END -
Henan released the latest prompt

In the past few days, Henan Province has continued to have high temperatures in ma...
The story of Zou Xu diagnosis room | Wet curb Yang Yu steaming sweat, dampness and relieving Yu Khan stop

Open column: [Zou Xu Diagnosis Room Story] The column is a special issue column le...