Focus on the latest research results of 2022 ASCO ASTRRRA, explore the important value of HR -positive breast cancer OFS assisted therapy before menopause
Author:Cancer Channel of the Medical Time:2022.06.16
*For medical professionals for reading reference
ASTRRRA is athy -based 2022 ASCO. What are the important revelations of the research results?
Breast cancer is the largest malignant tumor to threaten women's health. Among them, endocrine therapy is an irreplaceable important means for patients with hormone receptor -positive breast cancer. Selective estrogen receptor regulator (SERM) treatment has become an effective endocrine therapy for patients with early menstrual hormone receptor positive breast cancer in 5-10 years. Ovarian functional suppression (OFS) has been applied to breast cancer treatment for decades. Early auxiliary treatment studies have confirmed that the application of OFS alone can reduce the recurrence risk of patients under the age of 50 and improve survival. With the announcement of the 12-year follow-up results of evidence-based medical evidence such as Soft and Text, the results of the 12-year follow-up results, the results of ASTRRRA Studies, HOBOE-2 research, etc. It is more clinical benefits for patients with mid -to -high risks. The OFS joint plan has become the preferred plan for mid -to -high -risk patients recommended by major guidelines at home and abroad. At the American Clinical Oncology Society (ASCO) conference in 2022, Astrra studied 8 years of follow -up data [1], further verifying the trendy drug Gosherin combined with endocrine drugs for chemotherapy A significant treatment benefits brought by cancer patients.
The past and present life of Astrra research
Astrra is a phase III clinical study initiated by open labels, forward -looking, random, and multi -central researchers. There are two main purpose of research:
1. Assessing standards that he can further improve the disease -free survival (DFS) on the basis of the treatment standard for the treatment of Tam (DFS);
2. Evaluate patients with anesthesia or auxiliary chemotherapy caused by amenorrhea. After the recovery of ovarian function, the use of OFS can reduce the risk of disease recurrence.
The research group was ≤45 years old, and patients with breast cancer were added. Based on the 5 -year TAM standard therapy, I added OFS (Gosherin). The control group was TAM single medicine. It is patients with a menopause (evaluated every 6 months within 2 years) after chemotherapy, and the secondary of the secondary research includes the total survival period (OS) and tolerance.
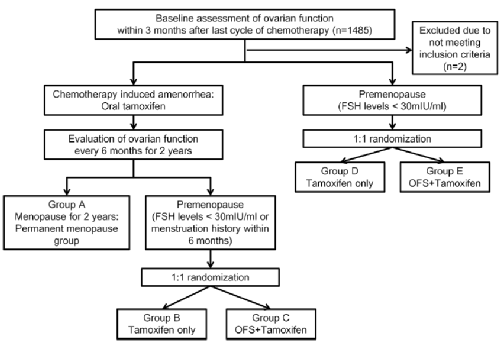
Figure 1.Astrra research design
01
ASTRRRA studies 63 months follow -up results
At the median follow -up of 63 months, a total of 1,293 patients were randomly assigned, of which 1282 patients met the analysis conditions. Among these patients, 635 were allocated to the TAM+OFS group and 647 were allocated to the TAM group. Starting from the time of the research group, the 5-year DFS rate of the TAM+OFS group was 91.1%, and the TAM single drug group was 87.5%(HR = 0.69; 95%CI, 0.48-0.97; P = 0.033). The 5-year OS rate of the TAM+OFS group is 99.4%, and the 5-year OS rate of the TAM single drug group is 97.8%(HR = 0.31; 95%CI, 0.10-0.94; P = 0.029). Patients who have not menopause or ovarian recovery have increased the OFS on the basis of TAM to significantly improve DFS [2].

Figure 2. DFS (left) and OS (right) beneficiaries (63 months of follow -up, starting from the research group time to evaluate)
02
ASTRRRA studies 8 years follow -up results
At the median follow -up of 106.4 months, the incidence of DFS events in the TAM+OFS group continued to decrease significantly. Starting from the time of the research group, the 8-year DFS rate of the TAM+OFS group was 85.4%, and the TAM single drug group was 80.2% (HR = 0.67; 95% CI, 0.51-0.87). 5.2%. The Asian group analysis of the patient's survival ending shows that in response to the Asian group patients at the age of 40-45, the 8-year DFS rate of the TAM+OFS group is significantly better than the TAM single pharmaceutical group (89.1%vs. 80.1%, HR = 0.5; 95%CI , 0.34-0.73), for HER2-negative patients, the 8-year DFS rate of the TAM+OFS group is also significantly better than the TAM single drug group (85.2%vs. 80.9%, HR = 0.7; 95%CI, 0.5-0.98).

Figure 3. DFS benefits of DFS in Research (8 years of follow -up, starting from the time of the research group)
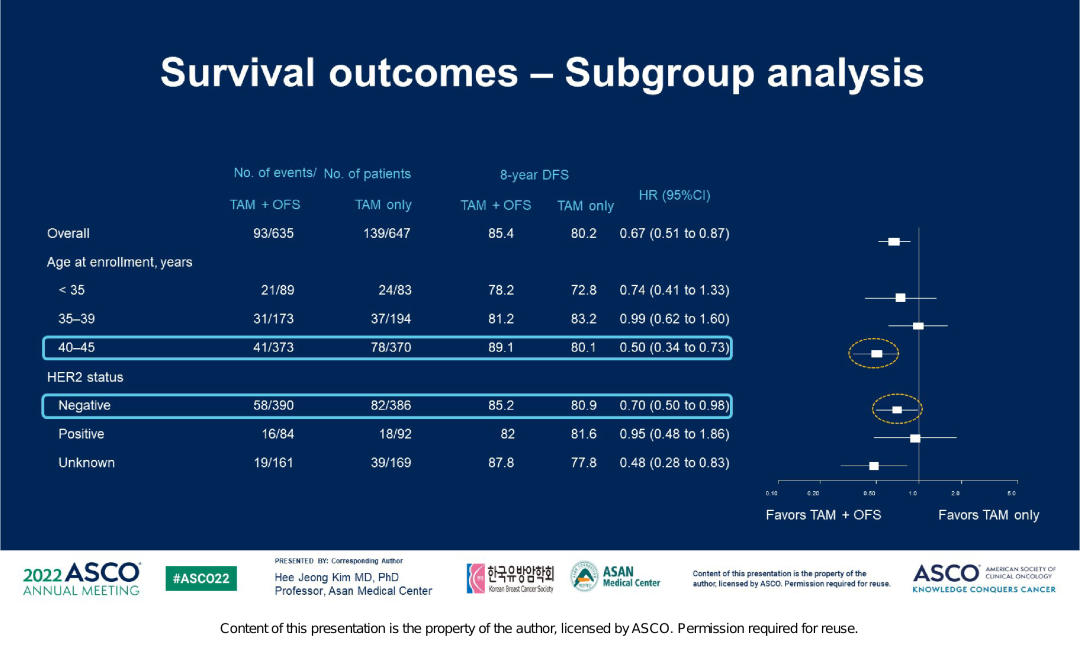
Figure 4. Asian group analysis of survival endings in the vastrra research
The TAM+OFS group still had 96.5%of patients in the 8th year of treatment. At present, the two groups of OS have no significant differences. The final OS data still needs to be fully evaluated for a longer time.
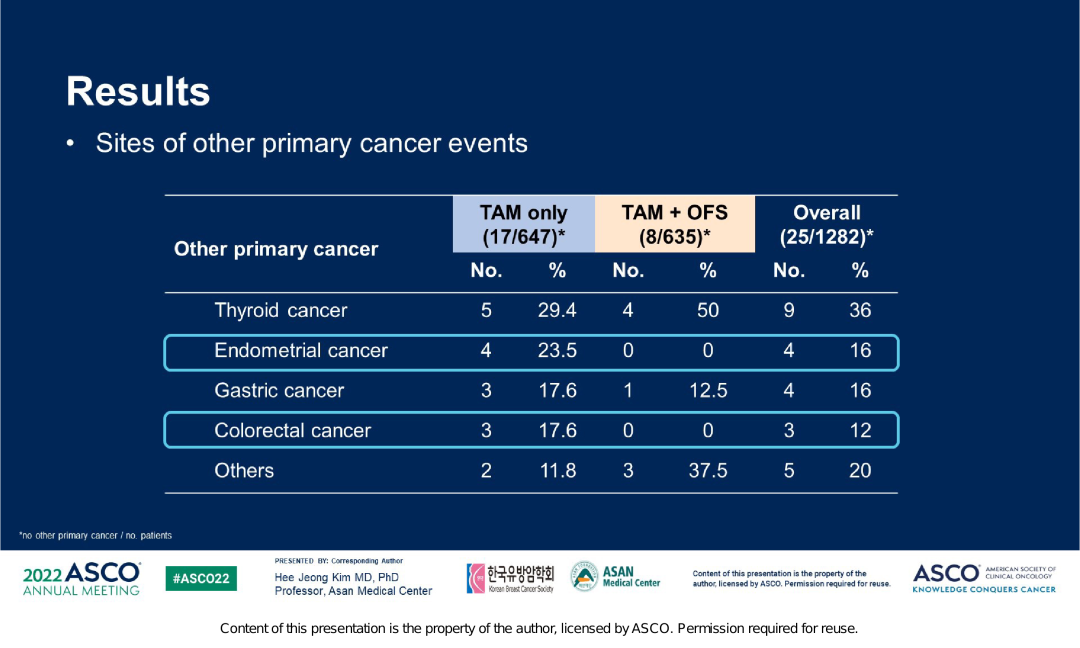
And in this study, the far -distance transfer rate of the TAM+OFS group is lower, with 8.5%and 12.2%. Given that Tam is manifested as weak estrogen, long -term use can cause a series of endometrial lesions and even cancer. And a clinical study of 74 280 patients with breast cancer (39411 cases of TAM groups, 3489 cases of 349 cases) showed that the incidence of endometrial cancer in TAM therapists was 38.8%, and patients who did not take TAM treatment were 19.8 %, It is prompted to increase the risk of endometrial cancer after taking TAM [3]. ASTRRA研究数据显示,TAM单药组子宫内膜癌的发生率更高,在所有原发第二肿瘤中占比23.5%,而TAM+OFS组的发生率为0,这也提示去势药物戈Shearin has a certain protective effect on endometrium. Figure 5. ASTRRRA study the incidence of second tumor in the Central Plains
Overall, the above data shows that over time, the survival benefits of patients continue to exist. The results of this study suggest that for patients who are still in a state of menopausal or ovarian function after chemotherapy, they should be considered to add OFS to TAM.
Astrra research brought the following revelation to the clinic
1. Hormone receptor -positive breast cancer before the mid -to -high risk is a significant beneficiary of the joint application of OFS
There are many studies on the basis of the addition of OFS on the basis of endocrine therapy on the prognostic prognosis of patients with positive breast cancer before menopausal hormone receptor. The article shows that of [4], of the 15,538 patients included in the 15538 patients, compared with OFS, compared with OFS, it can significantly improve the patient's DFS and OS, and reduce the risk of breast cancer on the side of the side.
The random and III phase SOFT study [5] carried out by the International Breast Cancer Research Group (IBCSG) also compared the treatment benefits of TAM+OFS and TAM single drugs. And it is worth noting that the sub -group characteristics of chemotherapy in SOFT research are similar to ASTRRRA studies, such as medium age (both two tests are 40 years old), lymph node positive ratio (ASTRRA is 55.0%, SOFT is 57.3%), and the organization is organized. Level 2-3 (ASTRRA is 75.5%, SOFT is 82.7%), Her2 positive (ASTRRA is 13.7%, and Soft is 18.1%). ASTRRRA's research data confirmed with SOFT's research results, that is, adding OFS after menopausal chemotherapy with sufficient recurrence risk can bring significant sustainable clinical benefits.
Regarding the beneficiaries of OFS, there are currently related recommendations at home and abroad. In the consensus of ST. Gallen in 2021 [6], the factors that consider recommended OFS include: high recurrence risk is still before menopause (94%) after chemotherapy. Phase II breast cancer (71%) in phase II. 2020 BCY4 Guide [7] (ESMO Fourth Edition Young Breast Cancer International Consensus Guide) Recommended for patients with higher recurrence risks should be combined with GNRHA based on SERM or aromatic enzyme inhibitors (AI); clinical installment I or stage I or Young women with stage Ⅱ breast cancer can not take SERM (due to taboos or adverse reactions for severe taboos), they can receive GNRH agonists, ovarian removal or AI+ Gnrha. The recurrence risk is high. Women who use TAM to restore ovarian function within 2 years after chemotherapy are completed after the end of chemotherapy, and GNRHA should be considered. In 2019, ESMO's early breast cancer guidelines are recommended [8]. For patients who need to receive chemotherapy and menstrual recovery after chemotherapy (especially in the first year, but accepted within the first two years), they should be strongly considered to add OFS in endocrine therapy. In 2016 ASCO's OFS Treatment Guide [9] pointed out that higher -risk patients should receive endocrine therapy containing OFS, and patients with low -risk patients do not need to use OFS endocrine therapy; It is recommended to receive endocrine therapy containing OFS; clinical stages of stage I or IIIIII consider higher -risk patients using chemotherapy, and consider endocrine therapy containing OFS.
In addition, "China Early Breast Cancer Ovarian function to suppress clinical application expert consensus (2021 edition)" [10] suggested that the mid -to -high -risk pre -tested hormone receptor positive breast cancer is recommended to receive OFS endocrine therapy; low -risk patients are recommended for selective estrogen Remnant regulators (SERM) single drug treatment; pre -menopausal patients who use aromatic enzyme inhibitors (AI) instead of SERM treatment need to be treated at the same time.
2. At present, authoritative guidelines tend to recommend the best treatment for GNRHA in the treatment of pre -menopausal breast cancer for 5 years
The previous important clinical studies of GNRHA for the treatment of pre-menopamental breast cancer were used for 2, 3, or 5 years of OFS treatment. For example, the treatment of GNRHA in ZIPP studies was 2 years, and the GNRHA course in ABCSG-12 studies was 3 years. The GNRHA course in Soft and Text is 5 years. The above treatments have confirmed the good security and tolerance of GNRHA [10]. Soft Study [5] shows that the 5 -year DFS, breast cancer survival rate and OS of the GNRHA combined TAM group reached 86.6%, 88.4%, and 96.7%, respectively. , 89.4%and 93.3%. The results of the HOBOE-2 study [11] show that 5 years of 5-year AI and OFS, 5-year AI union OFS, and 5-year TAM combination of OFS DFS were 93.3%, 93.2%, and 85.4%, respectively. Although ASTRRRA's research was only 2 years of treatment of OFS treatment, the crowd was included in the (new) auxiliary chemotherapy and did not menopher. Xifen's five -year OFS 2 years compared him for 5 years, significantly improved 5 years of DFS (91.1% vs 87.5%), and 5 years of OS (99.4% vs97.8%). Because there is no comparative study of different GNRHA treatment courses, the concept of extension of endocrine therapy and long -term follow -up results of SOFT/Text tests are recommended to assist GNRHA's standard treatment for 5 years. Patients who have completed 5 years of OFS standard treatment, the lack of research evidence of subsequent extension treatment. In 2021, the ST. Gallen [6] conference voted for this issue. Before menopausal state, 41%of the members of the experts recommended to continue OFS+AI, and 45%of the expert group recommended him to continue his Mosfen for 5 years. In 2021, the CSCO Guide For those who have completed the initial 5 years of OFS+AI for the first five years of treatment and good tolerance, it is recommended to use SERM 5 years or OFS+AI 5 years.
In 2015, the GNRHA treatment recommended by the "ESMO primary breast cancer diagnosis, treatment and follow-up clinical practice guidelines" [12] is 2-5 years, and "Guidelines and Specifications for Breast Cancer Diagnosis and Treatment of the China Anti-Cancer Association (2021)" [13 ] The recommended GNRHA treatment time is 5 years. ST. Gallen expert consensus [6], 2016 ASCO's guideline on OFS [9] update, and 2020 BCY4 guide [6] recommended treatment for 5 years. The "Early Breast Cancer Ovarian function inhibit the Consensus of Clinical Application Experts (2021)" [10] It is recommended that the standard treatment for GNRHA auxiliary endocrine therapy should be 5 years. After completing the five -year endocrine therapy of OFS, if you have not menopause and good tolerance, it is recommended to continue the 5 -year endocrine therapy or 5 -year SERM treatment for 5 years. Patients with low -risk selection of OFS for chemotherapy can consider OFS combined with endocrine therapy for 2 years.
Summarize
Astrra studies have shown that patients who are still in a state of menopausal or ovarian function after chemotherapy are still in the basis of standard endocrine therapy. Based on standard endocrine therapy, 2 years of OFS (Gosherin) can significantly improve the DFS of patients with hormonal positive breast cancer. The results of the research also provide new evidence -based on the choice of OFS beneficiaries and the best treatment. It is worth emphasizing that the current standard treatment of authoritative guidelines at home and abroad is 5 years. Patients with positive breast cancer benefits more.
Expert Introduction
Professor Wu Yan
Fudan University Affiliated Oncology Hospital, Deputy Dean, Chief Physician, Doctoral Tutor

Director of Drug Clinical Test Institutions
Chairman of the Breast Cancer Professional Committee of the China Anti -Cancer Association
Member of the Chinese Anti -Cancer Association soft tissue tumor committee
Chairman of the Breast Cancer Branch of the Shanghai Anti -Cancer Association
Deputy Chairman of the Shanghai Medical Association Tumor Target Molecular Specialist Branch
Member of the Shanghai Medical Association oncology Branch
references
[1] SOO Yeon Baek, et al. Adding Ovarian Function Suppression to Tamoxifen in Young Women withhormone-Sensitive Breast Cancer Who ReMenopausal or Resume
Menstruration after Chemotherapy: 8-year follow-up of the randomized
Astrra trial. 2022 asco. 506.
[2]Kim HA, Lee JW, Nam SJ, er al. Korean Breast Cancer Study Group. Adding Ovarian Suppression to Tamoxifen for Premenopausal Breast Cancer: A Randomized Phase III Trial. J Clin Oncol. 2020 Feb 10;38(5): 434-443. [3] Li Yuhe, He Yan, Yang Shuli, et al. Taking his influence on the endometrium after breast cancer [J]. China Practical Gynecological and Obstetrics Magazine, 2019, 035 (008 (008 (008 (008 (008 (008 (008 (008 (008 (008 ): 900-904.
[4] Bui K T, Willson M L, Goel S, Et Al. Ovarian SuppressionFor Adjuvant Treatment of Hormone Receptor-POSITIVE EARLY BREASTCANCER [J]. COCHRANE DTTABASE SYST Rev, 2020, 3: CD013538.38.
[5] Fraancis Pa, Regan MM, Fleming GF, Láng I, Ciruelos E, Bellet M, et al.Adjuvant Ovarian Suppression in PreMenopausal Breast Cancer. N English. 2015; 372: 436–46.
[6] Thomssen C, Balic M, Harbeck N, et al. St. Gallen/Vienna 2021: A Brief Summary of the Consensus Discostoming therapies for Wometh Early Breast Cancer 16 (2): 135-143.
[7] Paluch-shimon s, cardoso f, partridge a h, et al.ESO-ESMO 4th International Consensus Guidelines for BreastCancer in Young Women (BCY4) [J]. Ann Oncol, 31 (6): 674-6966.696.666.6666.6666.6666.66666666.666.6666666.6666.666.66966.
[8] Cardoso F, Kyriakides S, Ohno S, et al. Early BreastCancer: ESMO Clinical PractorIdelines for Diagnosis, Treatment and Follow-UP [J]. Ann Oncol, 2019, 30 (8) 120.20.
[9]BURSTEIN H J, LACCHETTI C, ANDERSON H, et al. Adjuvant endocrine therapy for women with hormone receptorpositive breast cancer: American society of clinical oncologyclinical practice guideline update on ovarian suppression[J].J Clin Oncol, 2016, 34 (14): 1689-1701.
[10] "China Early Breast Cancer Ovarian function to inhibit clinical application expert consensus (2021 edition)"
Ann oncol, 2018,29: viii704. [12] "ESMO primary breast cancer diagnosis, treatment and follow -up clinical practice guide"
[13] "Guidelines and Specifications of the China Anti -Cancer Association Breast Cancer Diagnosis and Treatment (2021)"
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
The seventh batch of national drug collection work was launched in early July

Original title: The seventh batch of national drug collection is intended to open ...
[Psychological Health] Gathering and Fung

Click to follow the official WeChat official WeChat of the Ministry of Public Secu...