The "Medicine King" battle is shocked and dangerous. Can K drugs reach the top? Can ADC become the next legend?
Author:Pharmaceutical economy Time:2022.08.05
In 2019, the research institution EvaluatePharma released a prediction report. By 2024, Merck's star anti -cancer drug Keytruda (Korida, commonly known as "K medicine") will replace Albervi's Humira (Mi Mira), becoming the new global " Medicine King ".
However, it seems that on the one hand, this prediction may be slightly conservative, and on the other hand, it also ignores the impact of the market structure brought by the new crown epidemic.
Earlier, overseas media released the "TOP20, the best -selling drug in the world in 2021". In 2021, Merlot fell from the throne of the "Medicine King". The annual sales were finally locked at $ 20.7 billion and retreated to the second place. The two other "players" of the top three are Pfizer's new crown MRNA vaccine, and Moderna's MRNA vaccine Spikevax.
The world's best -selling drug list in 2021
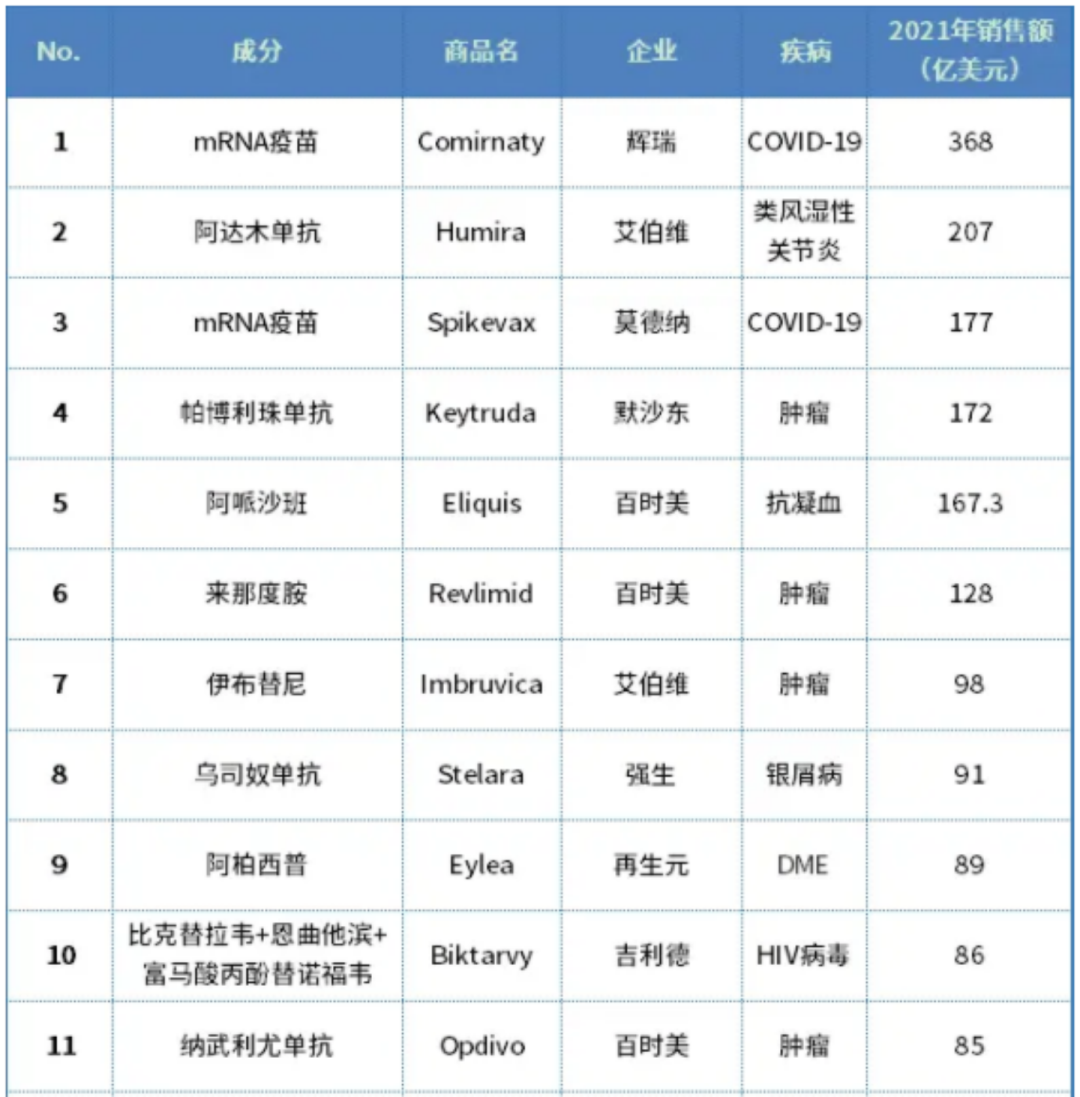
However, in addition to the new crown vaccine products with the epidemic, the sales of Meridodon K drug in 2021 have reached 17.2 billion US dollars, and it will once again become the world's best -selling anti -tumor drug.
Today, the impact of sales of the K -drug market towards the "Medicine King" continues. In the first quarter of 2022, the sales of Meridodong K medicine were US $ 4.809 billion, which had surpassed the US $ 4.709 billion, and touched the crown of the "Medicine King". US dollar sales defeated $ 5.252 billion in sales.
K -medicine and Xiu Meile you chase me. Even if you do not consider the new crown vaccine, the throne of Xiu Meile's "Medicine King" may be Yi Lord in 2022.
01
K medicine impacts "Medicine King"
Can high growth continue?
K-drug is an antitumor drug developed and produced produced by Meridodon. As a PD-1 antibody product that has long-term focusing on academic and industry, it has been expanded from R & D, clinical to listing, and indications. Attention.
In fact, the research and development project of K medicine has begun as early as 2003. In 2003, the ORANON team of the Dutch OSS company in Cambridge In order to treat an immune response self-immune disease that can treat an immune response such as rheumatoid arthritis such as rheumatoid arthritis. Immune response, obtained a PD-1 inhibitor. In 2007, Xian Lingya Company acquired the product from ORGANON. In 2009, Xian Lingya was acquired by Merck, and PD-1 officially entered the product library of Meridason.
Until 2010, Beltay Schimibao (BMS) unexpectedly discovered the amazing effect of immune drug tests, and Meridodon realized that PD-1 project's potential major business value was realized. In September 2014, K -drug was approved by the US FDA for patients with advanced malignant melanoma and officially became tumor immunotherapy drugs. In the following years, the scope of approval of K drugs continued to expand, and the number of applicable tumor types continued to increase.
At present, K drug has been approved by many indications around the world, including melanoma, non -small cell lung cancer, small cell lung cancer, head and neck squamous cell carcinoma, classic Hodg gold lymphoma, urinary tract cancer, gastric cancer, esophageal cancer cancer , Cervical cancer, liver cell carcinoma and other cancer species of multiple cancers, second -line, and multi -line therapy, and K drugs are still continuously conducting clinical trials to continue to expand indications. Each of the indications of K -drugs is approved, which means that it will bring further sales growth.

With many indications for the first layout, K drugs have steadily sitting in the "one brother" position in the current PD-1 market that are more competitive and launched against the global "Medicine King" throne.
On the other side, Xiu Meile has won the position of "Medicine King" from Liptet in 2012, and has won 9 years. However, as the patent period is approaching and the biological drug -like medicine enters the field, the era of fading is over.
It is understood that Xiu Meile patent will expire in 2023, and the biological pharmaceutical market has been smoky. Since September 2017, Albervi has signed an agreement with many pharmaceutical companies such as Anjin, Bolinger Yin Ge, Pfizer, Samsung Biology, Samsung Bioscope, and Samsuz. Pharmaceuticals enter the US market.
According to Ian Thompson, senior vice president and general manager of the US business, Anthth will launch the first biological generic drug in late January 2023.
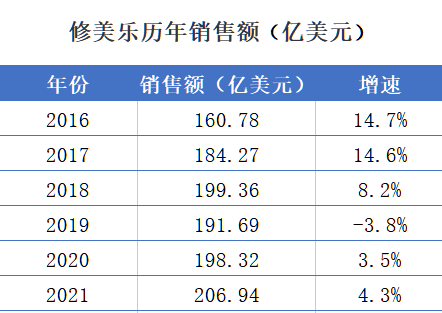
Although there are currently a variety of PD-1 inhibitors, they have been approved to be listed, but there are not many PD-1 drugs that can launch "head opposite" to K drugs.
However, K -medicine will not escape the patent cliff after all. It is understood that the core patent of K medicine will expire in 2028, which also means that in addition to ME-TOO drugs, K drugs will also usher in the market competition of biological drugs in the next few years. Can it continue to maintain high growth?
02
Looking for the new "moat"
Where is the successor of K medicine?
With the "low fruit" in the field of tumor immunotherapy, the many PD-1/L1 products, including K drugs, can only climb higher and steeper fields to conduct new explorations. This is also directly. Pushing up the risk of innovation research and development.
K -medicine seems to have encountered obstacles in the expansion of indications, and many expansion tests have been frustrated.
Recently, Meridon announced that K -drug has failed to achieve the main goal of the total survival (OS) and radiology no progressive survival (RPFS) in the clinical trial of some prostate cancer patients. Eliav Barr, senior vice president of Meridodon Research Lab, said: "We will continue to promote clinical development projects to evaluate K -drug -based combinations and new candidate drugs for treatment Patients. It is worth noting that this is the news of failure again in less than a month in less than a month. Previously, the K -Medicine Federation used Lenvima (Lenndininib) for the first -line treatment of hepatocytal carcinoma (UHCC) patients (UHCC) patient The main goal of the total survival period (OS) and the non-progressive survival (RPFS) of the LEAP-002 test in the third phase of the Leap-002 test, and the combined chemotherapy (CRT) for K-drug combined with Phase III Keynote-412 studies of patients with HNSCC (HNSCC) have not achieved the main endpoints of improving an incident-free survival (EFS).
Experts said that each product will go through such a "life cycle", long or short, typical product life cycle can generally be divided into four stages, namely the introduction period, growth period, maturity period, and recession period, different life cycles of the product, different life cycles of life cycle Need to create different moats. With many adaptive diseases, K medicine is treated in the market "one riding dust" in the market. In the future, it will set foot on the "Medicine King" throne. Essence
The success of any product is by no means accidental. K medicine once seized the huge dividend of tumor immunotherapy, and also made Merckon think: Where is the successor of K medicine?
Nowadays, Meridon is accelerating the development and acquisition of new drugs with potential. The signal released in the industry is very strong, or it is seeking performance growth points other than K -drug.
It is worth mentioning that the news that Meroson wanted to acquire ADC (antibody couplet drugs) company Seagen recently caused a global market sensation. Seagen is a leading enterprise in the field of global ADC drug research and development. It has long -term focus on the research and development of ADC drugs. Of the 14 ADC drugs currently on the market, 4 have cooperated with other companies with other companies. It is reported that in the subsequent pipelines of Seagen, more than 10 new ADC drugs are in different R & D stages.
The ADC project of Meridadon and the ADC project of Coron Pharmaceutical also aroused heated discussions in the industry. On July 26, Coronbutai will have an exclusive permission to Merhado with an exclusive permission to a certain clinical biomolecular tumor project with independent intellectual property rights. The proportion of net sales agreed on the two parties. Just two months ago, another ADC project in Columbal Botai had been included in the same way by Merhado, and the cumulative milestone payment was not more than 1.363 billion US dollars.
Fast layout in the ADC field and re -bet, Merck's "ADC ambition" is clearly visible. There are many innovative medicine tracks. Why did Merhado choose to force ADC?
In the industry's views, on the one hand, it is not easy to find new technologies and new products with potential to find potential. Astrakang and ADC drugs enhertu (DS-8201) from the first and third Communist ADC drugs have made it like Seagen. The target is more prominent; on the other hand, due to the unclear commercialization of CAR-T and gene therapy, layout ADC may be the best choice for Meridon.
Faced with the increasingly fierce competition pattern of the ADC market, can Merhado use ADC drugs to create the next "K medicine legend" in the future? It still takes time test.
Edit: Chen Shuwen
- END -
257 cases were added in 1 day!80,000 tourists stay!It was announced early in the morning: the city's restricted personnel flow

Emergency notice: From April 1st, Shenyang Metro has major adjustments!
Vaccine vaccination arrangement on June 8

The vaccine vaccination arrangement on June 8! The new crown vaccine strengthen...