CHART research debut at 2022ASCO, Chinese urinary tumor therapy towards a new level
Author:Medical newspaper Time:2022.06.08
At the annual meeting of the American Clinical Oncology Society (ASCO) in 2022, Professor Ye Dingwei, deputy dean of the Cancer Hospital of Fudan University and Director of the Shanghai Institute of Urology, made a verbal report to introduce the Research Research on the Riowuramine III- The latest progress of CHART has made Chinese urinary tumor innovative drugs from making Chinese voices on the international stage, marking the new steps of Chinese urinary tumor treatment. The results of the study show that the SHR3680 (Ritamamide) combined withrogens deprivation therapy (ADT) can significantly prolong the general survival period of patients with metastatic hormone sensitivity prostate (MHSPC), and significantly reduce the risk of patients' disease progress or death risk [1] It is expected to bring new treatment options for patients with metastatic hormone -sensitive prostate cancer (MHSPC).
The clinical needs that are not met in high incidence of prostate cancer are huge
Prostate cancer is the second common malignant tumor in men in the world. It is also the fifth cancer species of mortality. 14.1%of the total number of cancers and 6.8%of the total number of male cancer deaths. The incidence of prostate cancer in China is 15.6/100,000, and the incidence rate is increasing year by year. [2] Most patients have metastasized during the initial diagnosis and the prognosis is not good.
The growth of prostate cancer cells hasrogen dependencies. Due to the continuous activation of therogen receptor (AR) signal pathway, even if the patient receives the treatment of trending, it will inevitably develop into a trend of resistance to the prostate prostate prostate Cancer is more prone to metastasis, and the five -year survival rate of metastatic prostate cancer is less than 30%[3]. The metastatic hormone sensitivity prostate cancer (MHSPC) is mainly new endocrine therapy. The second -generation AR antagonist can effectively delay the time of entering metastatic removal resistance prostate cancer (MCRPC), extending the total survival of patients [4]. At present, there are only three types of second -generation AR antagonists approved by the US Food and Drug Administration (FDA), and there are still large unsatisfactory clinical needs.
SHR3680 (Revilunamide) is a new second -generation AR antagonist independently developed by Hengrui Pharmaceutical. Its innovative molecular structure introduces double hydroxyls to increase hydrophilicity and has higher plasma exposure And lower blood brain barrier. Studies I/II have shown that all doses of SHR3680 in the clinical trials of patients with MCRPC have anti -tumor activity [5].
Chart Study Data Research Data Research Prostate Cancer Show positive results
Chart Study [1] is a phase III clinical trial of international multi -center, random, control, and openness. Professor Ye Dingwei of the hospital led a total of 72 participating centers worldwide, including 22 European centers. The study aims to explore the efficacy and safety of SHR3680 combined with ADT comparison in patients with percaride combined with ADT in high tumor load MHSPC.
The main ending of the research is the shadow of the Evaluation of the Independent Review Committee (IRC)Such as learning non -progressive survival (RPFS) and overall survival (OS); secondary endpoints include RPFS evaluated by researchers, advanced prostate antigen (PSA) progress time, until the next bone -related incident (including fracture, spinal cord compression , Time for radiotherapy or surgery for bones), the start time, objective relief rate and safety end point for the next anti -prostate cancer treatment; the exploration end point includes the PSA response rate (defined as the PSA90 of the 12th weekend), and the PSA did not detect the rate Report the ending with patients.
In terms of images of no progressive survival (RPFS), as of May 16, 2021, the median follow -up time of the SHR3680 group and Bicaramide group was 22.1 and 20.4 months, respectively. The 24 -month RPFS rates evaluated by IRC were 72.3%and 50.0%, respectively. Compared with the Bikaramam group, the RPFS of the SHR3680 groups was significantly prolonged. The risk of imaging progress or death was reduced by 56%(Figure 1 1 To.
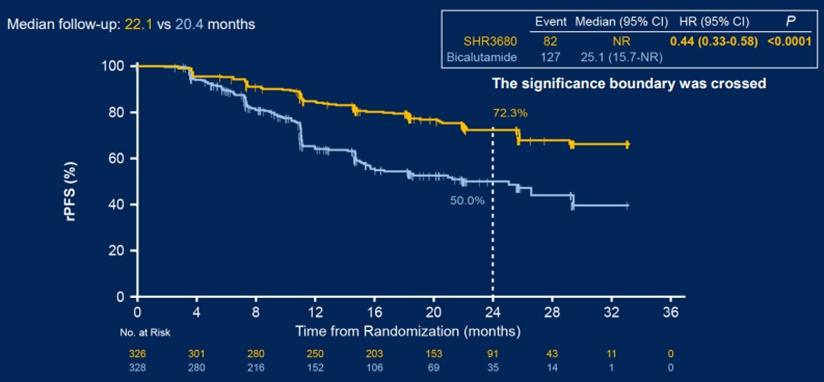
Figure 1 Analysis of the two groups of patients' RPFS comparison data as of: May 16, 2021
As of February 28, 2022, IRC The evaluation SHR3680 group and the median imaging of the Bicaramide group have no progressive survival (RPFS) data update. Compared with the Bikaramide group, the risk of imaging progress or death of the SHR3680 group of SHR3680 decreased by 54%.
In terms of overall survival period (OS), as of February 28, 2022, the median follow -up time of the SHR3680 group and Bicaramide group was 30.5 and 27.5 months, respectively, 24 months OS OS The rates were 81.6%and 70.3%, respectively. The overall survival (OS) of the SHR3680 groups was significantly extended, and the risk of death was reduced by 42%(Figure 2).

Figure 2 Analysis of the two groups of patients OS comparison data as of: February 28, 2022
In terms of ending, as of February 28, 2022, compared with the Bikaramam group, the RPFS evaluated by the SHR3680 group, the time to the PSA progress, the time for the next bone -related event, and the next anti -prostate cancer treatment began. The benefits of time are obvious. The objective relief rate of the SHR3680 group was 81.0%, which was 13 percentage points higher than that of the bibaramide group. In terms of exploration, the PSA90 in the 12th weekend of the SHR3680 group increased by 15.5 percentage points (94.4%VS78.9%) than thecaride group. Increased 35.2 percentage points (68.7%VS33.5%). During the follow-up period, the survival quality meter (FACT-P) survival quality score of the SHR3680 group was higher than that of the Bicaramide group (Figure 3).
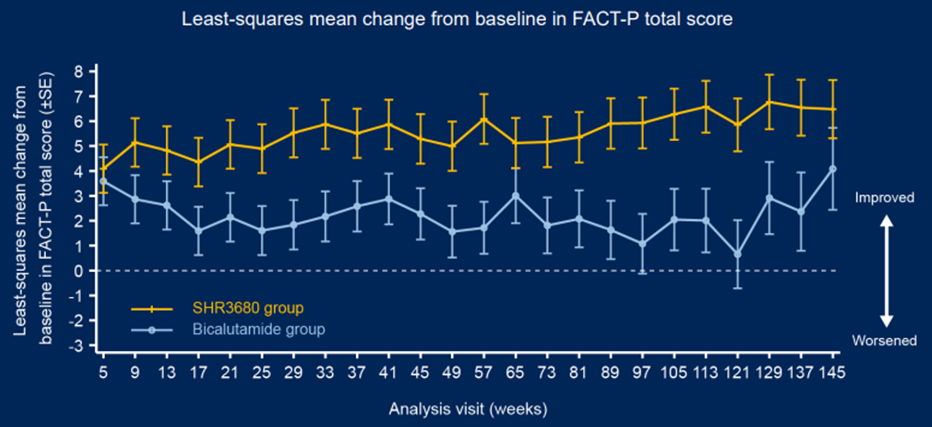
Figure 3 Fact-P survival quality scoring data during follow-up: February 28, 2022
In terms of security, the SHR3680 group has no treatment-related adverse events (Tra for Trape ) There was one case in the cause of death.
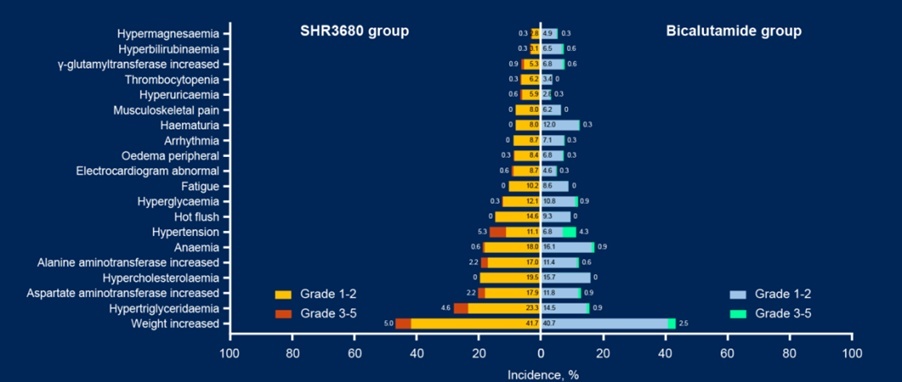
FIG. 4 TRAE statistical data during the follow -up rate of 5%as of: February 28, 2022
Chart research The results showed that the SHR3680 (Revilunamide) combined withrogens deprivation therapy (ADT) can significantly extend the general survival period of patients with metastatic hormone -sensitive prostate cancer (MHSPC), and significantly reduce the risk of disease progress or death of patients. The results of the clinical trials of Phase I/II are that the incidence of serious adverse reactions (AE), including fatigue and rash, is lower than other second -generation AR antagonists, and has no level of epilepsy, including fatigue and rash. occur. The success of Chart's research has more clinical guidance value for Chinese patients, filling the gap of China's independent research and development of second -generation AR inhibitors, and realizing a new breakthrough in the effectiveness and safety of the second -generation AR inhibitor. MHSPC's treatment provides new choices.
Focusing on the needs of Chinese patients to look at the international promotion of pharmaceutical innovation
It is worth mentioning that most of the patients included in Chart research are Chinese people, patients' physical scores, Gleason (Gleason) scores are more Poor, internal organs, more bone metastasis; and the control drugs used in this study are commonly used in Chinese clinical drugs that are widely used in Chinese clinicals in the treatment of MHSPC, and have more clinical guidance value for the Chinese population. This is also an important innovation research and development principle that Hengrui Medicine has adhered to for a long time, that is, closely focusing on the needs of Chinese patients, actively promoting the research and development of pharmaceutical innovation, and looking at international efforts to provide global patients with more and better treatment options.
It is reported that after the research of SHR3680 announced in July 2021, it was included in the breakthrough treatment variety by the Pharmaceutical Review Center of the State Drug Administration in August of the same year for the treatment of high tumor load MHSPC, then the listing application was qualified for priority review. In addition, another phase III study of SHR3680, that is, SHR3680 perioperative surgery for the treatment of high -risk prostate cancer, international multi -center, random, and control phase III clinical research, which was launched in September 2021. It is expected that domestic innovative drugs SHR3680 will bring new choices for patients with prostate cancer in the late China.
Reference information:
[1]. 2022 asco, oral abstract session 5005
[2]. Suung h, et al.
CA: a CANCER JOURNAL for Clinicians, 2021, 71 (3): 209-249
[4]. China Clinical Oncology Society (CSCO) prostate cancer diagnosis and treatment guidelines for diagnosis and treatment2021 version
et al. BMC Med. 2022 mar 4; 20 (1): 84
Types/Editor: Liu Zibo [123
Review: Chen Hui
- END -
alert!The General Administration of Customs issued important reminders
Multi -National Report Dengue Heating Epidemium According to the World Health Organization (WHO), San Domome and Princesby's outbreak of dengue fever epidemic, a total of 103 cases of heating the...
Henan issued emergency reminders in many places

Xinxiang issued a notice No. 26: Adjusting the normalization epidemic prevention a...