Medical insurance system officials were investigated; the price of domestic new crown pills was announced; byte -by -by -line acquisition of obstetrics and gynecology hospitals
Author:Kenji Bureau Time:2022.08.07
The domestic epidemic rebounded this week. As of August 7, 1140 new crown -infected cases reported in Hainan have reached 1140 cases, of which 827 were confirmed. Since August 7, Hainan will launch all the nucleic acid testing.
This week, the Astrakan New Crown Prevention in Boao, Hainan was officially commissioned to produce local production in China, and the drug was used for prevention before the exposure of the new crown virus.
The price of domestic new crown oral drugs is officially announced today: Azf's final price is 300 yuan per bottle. Compared with Phaxlovid's course of PAXLOVID, it has a price advantage for 2,300 yuan. This Friday, the real creature of Azfdin submitted a prospectus to the Hong Kong Stock Exchange ...
More information is sorted as follows:
Heavy policy
1. The two officials of the medical insurance system were investigated
News on August 3, the State Medical Insurance Bureau continuously released the news of the two officials in the body.
Ding Wenjun, director of the Office of the Office of the Medical Insurance Bureau (Reform Office), is suspected of serious violations of discipline and law, and is currently undergoing disciplinary review and supervision and investigation. In addition, according to the Discipline Inspection and Supervision Group of the National Commission for Discipline Inspection and the State Health and Health Commission and the Hebei Provincial Commission for Discipline Inspection: Qian Junjun, a commissioner of the Propaganda Information Department of the China Medical Insurance Research Association, is suspected of serious disciplinary violations and is currently undergoing disciplinary review and supervision investigation.
The China Medical Insurance Research Association is a non -profit community organization in charge of the State Medical Insurance Bureau. Its "China Medical Insurance" magazine was published in 2003 and is the only national academic journal in the country.
2.2 Pharmaceutical companies are rated as "serious" out of trust
On August 4th, the National Medical Insurance Bureau released the "Special Serious" and "Severe" dishonesty assessment results (No. 3), which was announced from April 1st to June 30th, 2022. There are two pharmaceutical companies:
One is Yunnan Tongfeng Pharmaceutical Co., Ltd., because the company gives a rebate or improper benefit to the relevant personnel of the Diqing Shangri -La People's Hospital, so that the drugs they operate can get additional trading opportunities, competitive advantages and sales quantities, which is equivalent to RMB 600,000 Yu Yuan was rated as "severe" business;
Another is Ningxia Minning Pharmaceutical Co., Ltd., which is a rebate or improper benefit to the relevant personnel of the People's Hospital of Guyuan City to obtain additional transaction opportunities, competitive advantages and sales quantities, which is equivalent to 1.14 million yuan Yu Yuan was rated as a "serious" business.
3. The direct settlement rate of hospitalization expenses will increase significantly
On August 3, the official website of the Medical Insurance Bureau disclosed the related matters of inter -provincial medical settlement: at the end of July this year, the National Medical Insurance Bureau jointly triggered the "Notice on Further Direct Settlement of Basic Medical Insurance Cross -Provincial Different Places", unified, unified The cost of direct settlement of slow specialties for hospitalization, ordinary outpatient and outpatient clinics in my country.

According to the "Notice", by the end of 2025, the direct settlement rate of hospitalization expenses across provinces will be increased to more than 70%. Related treatment costs are gradually incorporated into the scope of cross -provincial direct settlement. The regulation of medical treatment in different places is convenient. Basically, the medical insurance reimbursement can be reimbursed online and offline.
Industry affairs
1. Domestic new crown oral medication per bottle does not exceed 300 yuan
On August 7th, according to Henan Daily, the first domestic oral new crown drug Azf's fixed price disclosed by the first domestic oral new crown medicine developed by the real biological biology disclosed: 1 mg per bottle, each bottle price of no more than 300 yuan.
In July of this year, Azf was approved by the Conditions of the Pharmaceutical Supervision Bureau to treat adult patients with ordinary types of new crown pneumonia. Fosun Pharmaceutical issued an announcement on the same day that Azf's exclusive commercial rights and interests were obtained. Before Azfding, the only new crown oral medicine in China was Paxlovid of Pfizer. The price was 2300 yuan a course of treatment, and it has been included in medical insurance. Compared with the two, Azf has a price advantage.
Three days before Azf's final price disclosure, the real creature officially submitted a prospectus to the Hong Kong Stock Exchange.
2. Beijing one nucleic acid laboratory is forcibly executed
On August 4th, according to Tianyan Check Information, Beijing League Medical Inspection Labs Co., Ltd. and its legal representative Wang Xuegang and other newly executed person information. The executive court is the Beijing Haidian District People's Court, with an implementation amount of more than 5.04 million yuan Essence
In May this year, due to illegal regulations, "5 mixed 1" and "10 mixed 1" mixed inspections were performed on the Beijing area in violation of regulations. The Beijing police filed a case for investigation, and 17 people, including the legal representative, were criminal measures by the Haidian District Police in accordance with the law.

3. Colom Pharmaceutical and MSD reached a $ 2.2 billion cooperation
On August 5th, Colom Pharmaceutical disclosed new progress with Merosa East.
The first cooperation was that in May, Kelun Pharmaceuticals had a paid exclusive permission to the research, development, manufacturing, and commercialization of its biomolecular tumor project A (SKB264, Trop2 ADC) to the MSD. As of the announcement, Columbia has received a license fee of $ 30 million paid by MSD. According to the agreement between the two parties, the follow -up Coron is expected to receive the development, scientific research and various milestones provided by MSD more than $ 1.3 billion.
The second cooperation is that in July, Kelun Pharmaceuticals biomolen tumor project B, which has independent intellectual property rights in July, to study, develop, manufacture and commercialize MSD globally globally. According to Coron's announcement, in the third quarter of 2022, Colom will receive a $ 35 million down payment and a total of 901 million yuan in various milestones. If the progress is smooth, Coronfang will receive more than $ 2.2 billion from MSD, and will also receive two commercialized sales of two products.
4. Byte beating acquisition of the United States and China Yihe
News on August 5th, byte beating acquisition of Beijing Meizhongyi and Medical Management (Group) Co., Ltd. The United States and China are a high -end private maternal and children's hospital. As of now, there are 7 women's children's hospitals, 2 comprehensive outpatient clinics and 5 confinement centers.
5. Pharmaceutical Biology and Astraikon cooperate to produce neutralized antibodies
On August 4th, Yaoming Bio announced that the new crown prevention and antibody combination drug Eno reached a strategic cooperation with Astrikon's new crown prevention.
It is understood that Astrakon's new crown prevention of neutralized and antibody combination drugs landed in Boao, Hainan in June this year, and used it for the new crown virus before exposure. As of 2021, the primary liquid produced by Yaoming Bio has nearly 50%of Astraon's global vaccine supply.
New drug inventory
1. Changchun High -tech Reorganization Human growth hormone injection was approved
On August 4th, Changchun High -tech issued an announcement saying that the State Drug Administration has issued a certificate of reorganized human growth hormone injection related drug approval documents. The newly added indication is a specialty shortness (ISS).
2. Hengrui Pharmaceutical Breast Cancer New Phase III clinical success

On August 1st, Hengrui Pharmaceutical announced that it had the first -line therapy of HER2 positive recurrence/metastatic breast cancer in the Malaccitanine pyrolinine tablets in Malaysia Phase III clinical study of parallel control and multi -center reaches the end. The company will submit a listing application to the Pharmaceutical Supervision Bureau in the near future.
According to the announcement, piperinib is a small molecule, irreversible, and pan Erbb receptor tyrosine kinase inhibitors. In 2021, the global sales of related products were about 687 million yuan.
3. Anshin 3.7 billion US dollars was used for immune disease medication
On August 4, Anjin announced that it would acquire ChemocentryX for about $ 3.7 billion. It is understood that enough acquisitions, Angin Company will get a First-in-Class 5A receptor (C5AR) inhibitor of CHEMOCENTRYX company-Tavneos.
The drug was approved by the FDA in October 2021, and the use of standard therapy as a severe active ANCA -related adult patients with adult patients. In the first quarter of 2020, TAVNEOS sales in the United States reached $ 5.4 million.
Writing | Nicotinamide
Edit | Jiang Yun Jia Ting
Operation | Valley
Illustration | Visual China
### Byte Beat ###
- END -
The three -dimensional "blockbuster" in the operating room, 3D fluorescent navigation helps doctors for precise surgery
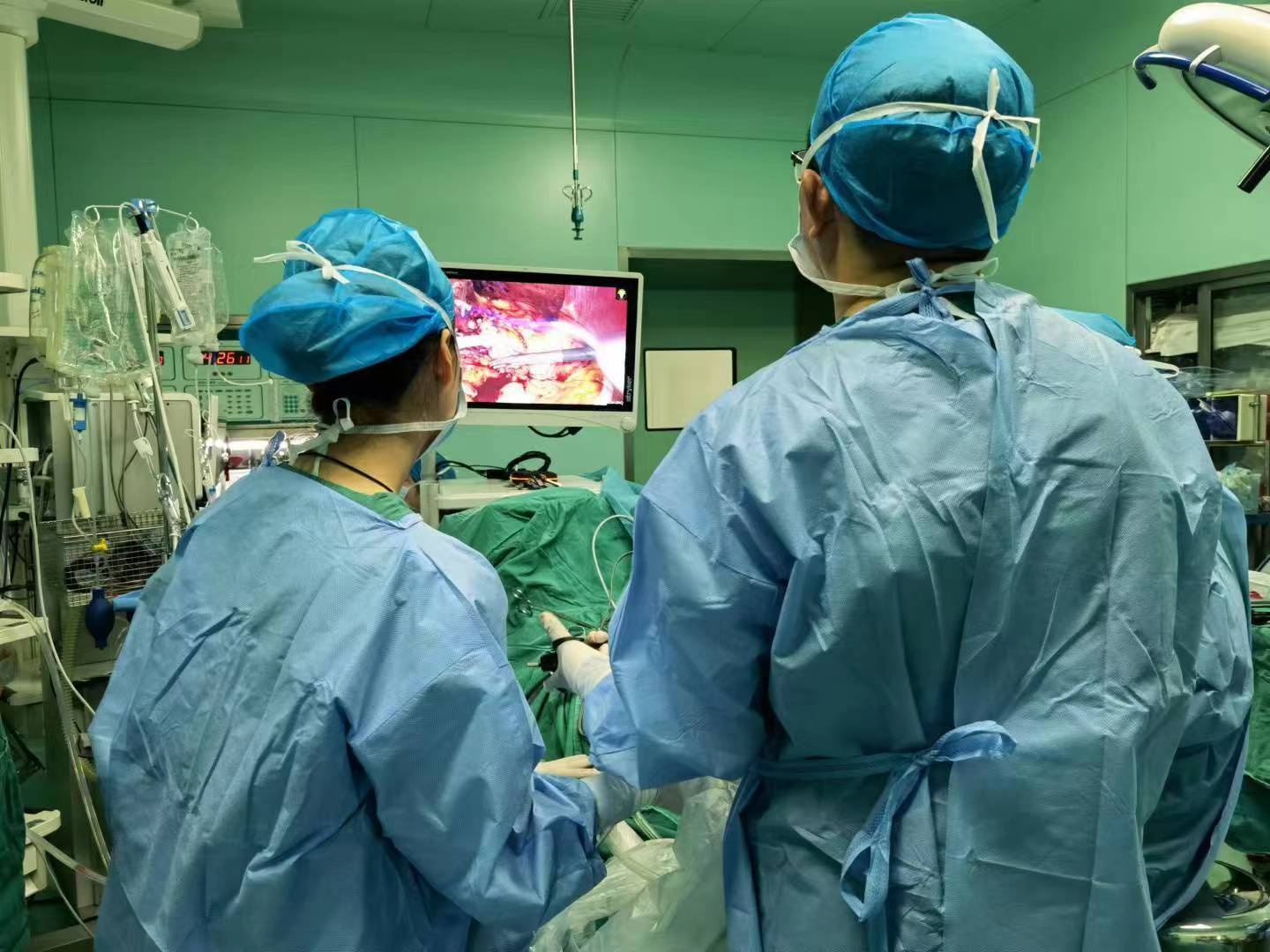
The Yangtze River Daily Da Wuhan Client August 8th (Correspondent Ma Yaoyao) Recen...
90 -year -old music naughty boy to prevent cerebral thrombosis in 50 years to eat 10,000 onions!"Live 100 years old" unlock fancy health care method

90 -year -old music naughty boyPrevention of blood vessels to eat it!The 90 -year ...