Make shortage of medicines without shorts!The latest post of four departments
Author:Sichuan Observation Time:2022.08.10
Recently, the Ministry of Industry and Information Technology, the National Health and Health Commission and other four departments jointly issued a notice to deploy the monitoring of drug production reserves and reserve monitoring in the centralized procurement of national organizations. what does that mean? Will there be a shortage of shortage drugs in the future?

In the data map, Xinhe reporter Zhang Tianfu Photo Data Map Chinese News Agency reporter Zhang Tianfu Photo
What is a shortage of drugs?
In 2020, the "Notice on Printing and Distributing the List of National Short -State shorts" issued by the National Health and Health Commission, the Development and Reform Commission, and the Ministry of Industry and Information Technology shows that there are 6 varieties of national shortage drugs, which focus supply.
In addition, there are 57 varieties in the national clinical must be prone to shortage drugs, focusing on clinically necessary and irreplaceable or unable to replace the risk of supply shortage. , Prevent shortage.
It is worth noting that the catalog of the variety and monitoring enterprise directory of the shortage drug monitoring varieties have been implemented.
The notice of the four departments also pointed out that in terms of monitoring varieties, the national shortage drug list varieties announced by the National Health and Health Commission, the national clinical must be prone to shortage of drug key monitoring list varieties, and the National Organization of Drugs Organized by the State Medical Insurance Bureau. Dynamic adjustments to monitor the catalog.
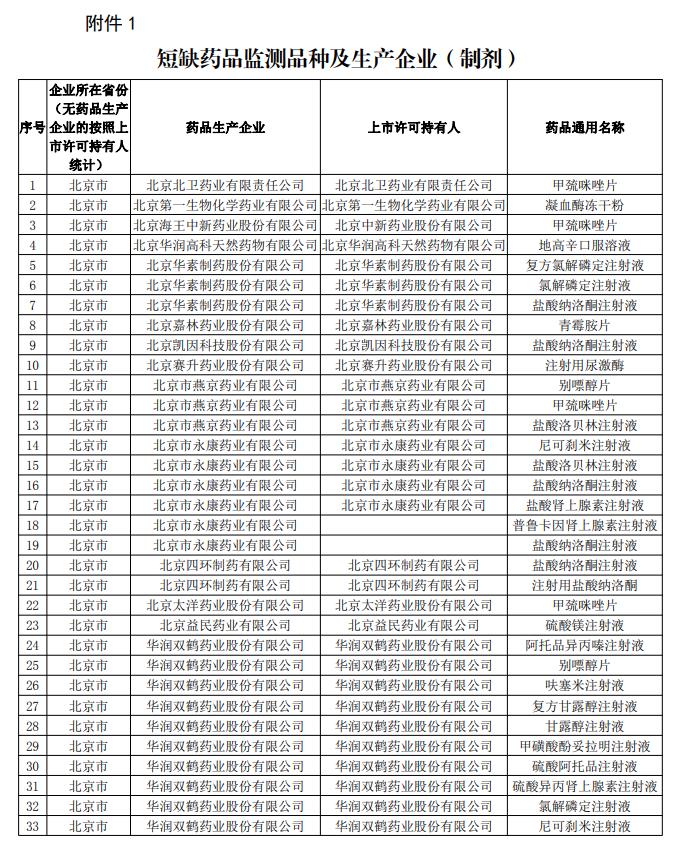
Screenshots of the "shortage drug monitoring varieties and production enterprises (preparations)" in the notice. Screenshots of the "shortage drug monitoring varieties and production enterprises (preparations)" in the notice.
In order to make the shortage of medicines no shortage, what have been done over the years?
In fact, in order to ensure the supply and production of shortage drugs, many departments have issued relevant measures in recent years.
In 2021, the General Office of the State Council issued the "Fourteenth Five -Year Plan" National Medical Security Plan. It proposes to improve the shortage of drug monitoring and early warning and hierarchical response system, increase law enforcement for illegal acts such as the monopoly of raw and drug, and further do a good job of stable prices for shortage drugs.
The plan is clearly established to gradually establish a system of emergency reserves, inventory and capacity reporting system for winning bidding manufacturers to ensure centralized procurement of drug supply. Support pharmacy chain, professionalization, and digital development, and better give full play to the unique advantages of pharmacies and pharmacists. Relying on the national unified medical security information platform to support the circulation of electronic prescriptions.
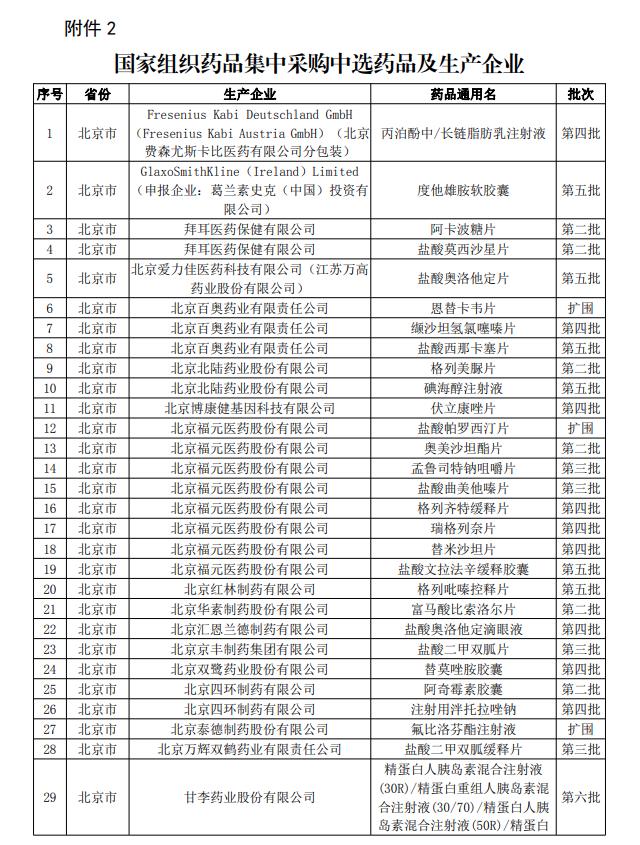
Screenshots of the "National Organization of Pharmaceuticals and Production Enterprises". Screenshots of the "National Organization of Pharmaceuticals and Production Enterprises".
In November 2021, the State Drug Administration developed and constructed the information collection module of the production supply and production report of shortage drugs in the drug information collection platform.
In January of this year, the "Fourteenth Five -Year Pharmaceutical Industry Development Plan" jointly released by the Ministry of Industry and Information Technology, the Development and Reform Commission, and other nine departments proposed to enhance the ability to ensure the supply of drug supply. Focusing on basic drugs, children's medicines, urgent rescue drugs, etc., improve the purchase and payment policy of prone to shortage, and timely include qualified varieties to be included in hanging network procurement in time to mobilize enterprises to produce enthusiasm. Dynamic adjustment of the national shortage drug list and clinical clinical must be prone to shortage of drug key monitoring lists, strengthen the production and supply chain monitoring and early warning of prone to shortage drugs, and establish a platform for supply and demand and demand for prone to shortage. Support the development of drug supply guarantee consortia, expand the coverage of small variety drugs (shortage drugs), deepen the supply chain collaboration, and promote the integrated development of key varieties and drugs and preparations.

Data Map Guo Jia Photo Data Map Guo Jia Photo
In the future, these situations may be interviewed or rectified
In addition to the above -mentioned measures, how to monitor the shortage of shorts in time has also been answered recently.
On August 9, the Ministry of Industry and Information Technology's website disclosed the notice of the four departments of the shortage of drugs and the selection of drug production reserves in centralized procurement of national organization drugs.
Among them, it requires to give full play to the role of provincial -level business linkage mechanisms, further strengthen information Unicom sharing, strengthen monitoring and early warning, improve the management measures for the grades of shortage drugs, guide the supervision of enterprises to fulfill the obligations of information and fill in, and continuously improve the ability of drug production and supply and guarantee capabilities. It is better Meet the health needs of the people.
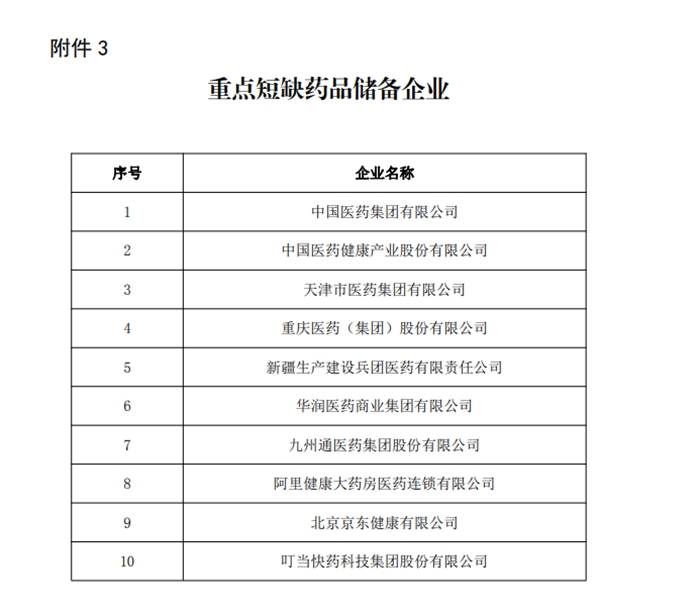
Notice announced key shortage drug reserve enterprises notified the key shortage drug reserve enterprises announced
The notice clearly states that before the 10th of each month, the shortage drug production enterprises and the selected pharmaceutical manufacturers in the collection, through the "shortage drug production supply monitoring and early warning platform", fill in the "Monitoring Report on the Production and Supply of Drug Production and Supply of Pharmaceutical Enterprise Enterprise", "National centralized procurement of drug production production Supply Monitoring Statement; key shortage of drug reserve enterprises through the "National Medical Reserve Management Information System" to fill in the "Statistics Form for the Inventory of Short Poseida".
The notice requires that the shortage drugs and collection of drug production enterprises are the first responsible person for the reporting of production reserves monitoring information. It is necessary to establish a sound monitoring information reporting system; specify the real, accurate, complete, and timely reporting production and reserve data. Late reports and refusal; cooperate with relevant departments to investigate the shortage of drugs, provide the information required for the investigation; set up the production and supply stock safety warning line and make a good response plan.
The notice also requires strengthening supervision and management. The competent department of local industry and informatization, together with the relevant departments, is responsible for the monitoring of the production reserve monitoring of drugs and selected drugs in the region, organize enterprises to submit relevant data, review the integrity and accuracy of the data, and strengthen the supervision and inspection of the quality of drugs. Supervise the production enterprises to implement the responsibility for supply and discontinued reporting in accordance with the procurement agreement. For enterprises that have not been implemented as required, interviews with each other and requires rectification within a time limit.
Sichuan Observation (Source: China News Network)
- END -
[Campaign to rectify pension fraud] 3.8 yuan to buy 30 eggs?The old man's map discount, the counterattack of 270,000 retirement money

In order to deeply implement the people -centered development of the people and ac...
As of 24:00 on August 18th, the latest situation of Jiangsu's new coronary virus pneumonia epidemic
According to the authority of the Jiangsu Provincial Health Commission on August 19:At 0-24 on August 18th, 1 new local diagnosis case in Jiangsu (Nanjing report, which was returned to the Soviet Unio