New PD-L1 inhibitor, ADC ... New strategy of first-line lung cancer treatment | 2022WCLC
Author:Cancer Channel of the Medical Time:2022.08.15
*For medical professionals for reading reference

Directly hit WCLC, the medical industry takes you to see new progress in NSCLC's front -line treatment!
The research and development of lung cancer targeted and immune new drugs presents a well -spray development. Therefore, how to make NSCLC patients maximize benefits during the first -tier treatment, and then allow patients to survive for a long time and become the focus of academic research. From August 6th to 9th, 2022, at the 2022 World Lung Cancer Conference (WCLC) in Vienna, Austria, the Cancer Research Association (IASLC) showed the corresponding research results and let us see it quickly.
AVELUMAB VS Chemotherapy for First-Line Treatment of Advanced PD-L1+NSCLC: Primary Analysis from Javelin Lung 100.
Summary: OA15.03
Javelin lung 100: Compared
AVELUMAB is a monoclonal resistance of anti-PD-L1, which shows the activity of anti-tumor and acceptable security in NSCLC's treatment. Professor M.Reck's team conducted an open label and a multi-center Javelin LUNG 100 Study, with a view to clear AVELUMAB single medicine (two dose schemes). Effectiveness and safety [1].

2022WCLC Abstract Screenshot
Researchers were included in a total of 1214 PD-L1 positives (PD-L1 expression ≥1%) and EGFR/ALK's unprepared transfer or recurrence (M/R) patients with wild types. At a ratio of 1: 1, it is randomly divided into AVELUMAB 10mg/kg every 2 weeks (Q2W) and platinum dual drug chemotherapy group [once every three weeks (Q3W)], and layered according to pathology. Subsequently, after revising the plan based on the pharmacokinetics and exposure analysis, the patient was randomly divided into Avelumab (10mg/kg) Q2W group, platinum dual pharmaceutical chemotherapy Q3W group, and the Avelumab QW group (10mg of 10mg/kg) according to a ratio of 1: 2: 2 After 12 weeks of/kg qw, the 10mg/kg Q2W scheme was administered). At this time, it was expressed according to the historical and PD-L1 expression (height ≥80%; moderate ≥50%; any ≥1%) layer. The main end of the study is the Independent Censors (IRC).
The assessment of the general survival period (OS) and the survival period of non-disease progress (PFS) of the evaluation PD-L1 high expression (OS), and the results show:
The patient distribution showed 366 cases in the AVELUMAB Q2W group, 526 cases of platinum -containing dual drug chemotherapy group, and 322 cases in the Avelumab QW group. As of October 2021, the follow -up time has exceeded 41 months;
There are no significant differences between OS and PFS between the AVELUMAB group and the platinum dual drug chemotherapy group (Figure 1);
Among the patients with high PD-L1 high-expression, the AVELUMAB Q2W group, Avelumab QW group, and platinum-containing dual medicinal group received the proportion of anti-PD-1/L1 monoclonal treatment after the research was over, respectively. %;
The AVELUMAB Q2W group, Avelumab QW group, and platinum -containing dual -drug chemotherapy groups were 95.8%, 96.6%, and 96.8%, respectively, and the proportion of adverse reactions of levels ≥3 were 60.1%, 56.9%, and 56.9%, respectively. 64.8%;
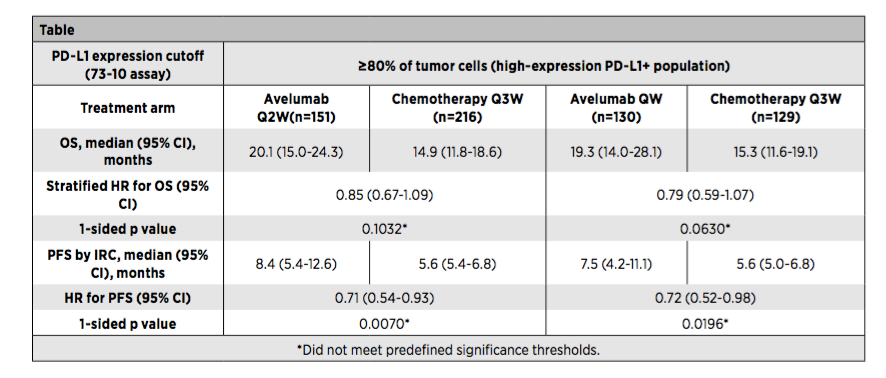
Figure 1: Javelin LUNG 100 Study PD-L1 high expression crowd efficacy results
In short, the IRC evaluation of the Javelin LUNG 100 studies did not show that Avelumab (Q2W or Q1W) compared to Platinum dual-containing chemotherapy in PD-L1 highly expressing the advantages of OS and PFS in patients with NSCLC. Safety results are consistent with previous research results.
MA13.07 Tropion-LUNG02: Initial Results for Datopotamab DeruxTecan Plus PEMBROLIZUMAB and Platinum Chemotherapy in Advanced NSCLCC
ADC Drug Datopotamab Deruxtecan combined immunohistochemical point inhibitor + chemotherapy treatment advanced NSCLC is quite prospective
Most NSCLC patients will recur after 8 to 10 months after the start of the front line. Therefore, new treatment methods need to be urgently needed to meet the treatment needs. Datopotamab DeruxTecan (DATO-DXD) is the antibody coupling drug (ADC) of Trop2. Single drug treatment shows encouraging activity and management security in the treatment of recurrence/refractory (R/R) in advanced/metastatic NSCLC treatment. Sex [NCT03401385, 6mg/kg for treatment, the objective relief rate (ORR) is 28%, and the duration of duration (DOR) is 10.5 months]. In addition, clinical studies have shown that Dato-DXD combined with PD-1 monoclonal anti-anti-anti-resistance has a more tumor effect compared to the two. Tropion-LUNG02 studies to clarify the initial efficacy and safety of Dato-DXD combined with Paborzab ± chemotherapy in NSCLC treatment. 2022WCLC Abstract Screenshot

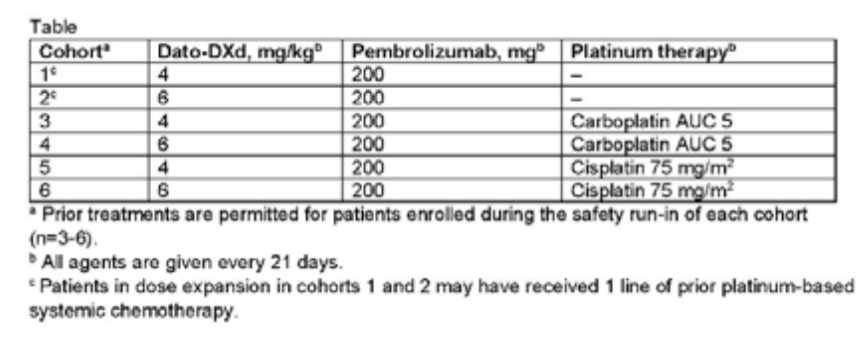
Researchers set up a total of 6 queues, each of which was about 20 patients (Table 1). Unless otherwise explained, the patients in the expansion queue were patients with advanced / metastasis NSCLC patients who had not been treated in the past. The main goal of the study is to evaluate tolerance and safety, and the secondary goal is to evaluate the efficacy, pharmacokinetics and antibody antibodies. As of January 2022, a total of 60 patients were treated. The median age of patients was 64 years old, 35%of patients PD-L1 expression was <1%, and PD-L1 expression was 1%to 49%and PD-PD- The proportion of patients with ≥50%of L1 expression was 25%, and the remaining 15%of patients with PD-L1 expression state was unknown. research shows:
The duration of median treatment is 2.7 months, and 67 % of patients are still being treated;
In terms of safety, 10%of patients stopped treatment due to adverse reactions. 20%of patients were reduced when using DATO-DXD. Adverse reactions are stomatitis (42%), nausea (38%), and fatigue (27%).
All combined therapy groups are considered tolerant treatment and can be converted to dose extension queues;
Among the 46 patients with appraisal therapy reactions across the queue, 39%of ORR [9 cases were partially relieved (PR), 9 cases were transformed into PR, the curative effect was to be confirmed], the disease control rate (DCR) was 82.6%;
Among the 16 patients who can evaluate the effective first -line treatment, the ORR is 69%(5 cases have been confirmed as PR, and 6 cases are being transformed into PR, the effect is to be confirmed), and the DCR is 100%.
Table 1: Treatment combination and scheme of 6 queues
In short, in the NSCLC frontline and or recurrence / difficulty in the treatment of backline, Dato-DXD combined with Pabberzetsu Mipidum ± platinum chemotherapy showed the tolerance safety and significant anti-tumor activity. According to researchers, the research is the first study in NSCLC patients, using DATO-DXD combined with Paborizumab ± platinum chemotherapy.
references:
[1] M.Reck, et al.OA15.03.2022WCLC.
[2] B.Levy, et al.MA13.07.2022WCLC.
The first release of this article: the medical world tumor channel
Author: Tumor Baby
Editor in charge: Sweet
- END -
Create the door and windows of the air conditioner will cause indoor carbon dioxide concentration to exceed the standard?

After entering, the weather is stuffy and humidThe use frequency of air conditione...
alert!Monkey acne cases have entered Asia

The Ministry of Health of Singapore said on June 21 that the first case of monkey ...