New progress has been made in brain metastaset therapy, biomarkers ... ROS1+lung cancer diagnosis and treatment!丨 2022 asco
Author:Cancer Channel of the Medical Time:2022.06.17
*For medical professionals for reading reference
ASCO ROS1+Latest Progress List
The annual American Clinical Oncology Society (ASCO) Annual Conference was held on June 3-7, bringing cutting-edge and authoritative academic feasts to doctors and practitioners in the field of cancer diagnosis and treatment. Lung cancer is one of the most threats to the health and life threats in the world, and it is also the main reason for the mortality of cancer -related diseases worldwide. Therefore, the diagnosis and treatment of lung cancer is particularly concerned, and targeted treatment is an indispensable part of it.
With the discovery of a variety of drive gene mutations such as EGFR, ALK, MET, NTRK, ROS1, targeted therapy for related targets is also continuously developing. (PFS) or General Survival (OS) has been improved.
This article will share the latest clinical trial results and messages of ROS1+NSCLC at the ASCO meeting ~
1
BFAST research: late -stage blood detection ROS1+advanced/transfer NSCLC, Ensteinib the treatment is safe and effective
At present, the detection of biomarkers of tumor tissue provides a basis for the formulation of tumor treatment plans. However, the tumor tissue biopsy may have inadequate testing and may have risks in patients with advanced/metastasis NSCLC. External blood testing can overcome these problems, but the value of the guiding clinical practice needs to be further confirmed.
BFAST (NCT03178552) is a clinical study of global open labels and multiple queues. It explores the recruits of CFDNA sequencing detection drive genes for advanced/transfer NSCLC, and uses the results of the treatment plan for the effectiveness and safety of the treatment plan. The results of the ROS1+queue were treated with Ensteinib therapy. (Summary number: LBA 9023)
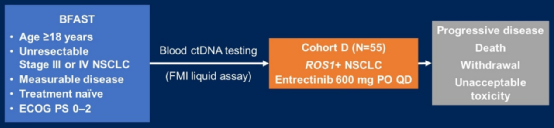
55 patients with ROS1+(patients with no brain metastases) who were admitted to the group received orally at the Er Entininib 600mg/day, until the disease progressed or unacceptable toxicity, drug discontinuation or death. The main endpoint is the objective relief rate (orr) (RR) (RR) (RR) (RR); the secondary end point is the continuous relief time (DOR) and PFS evaluation of INV evaluation, and the ORR, DOR, PFS of the Independence Jury (IRF) evaluation (IRF) evaluation (IRF) , OS, Central nervous system (CNS) progress time and security. The patient's baseline is as follows:
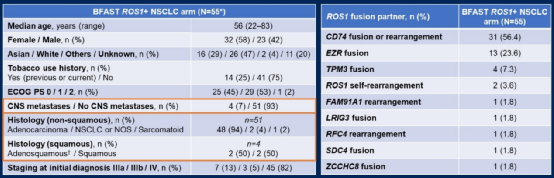
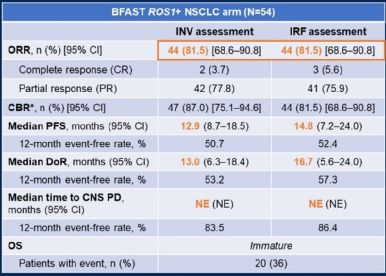
As of November 26, 2021, among the 54 patients with appraisal, ORR was 81.5%(95%CI 68.6%-90.8%). The median Dor of INV evaluation is 13.0 months (95%CI 6.3-18.4 months), and the median PFS is 12.9 months (95%CI 8.7-18.5 months); the median Dor evaluated by IRF is 16.7 months ( 95%CI 5.6-24.0 months), the mid-position PFS is 14.8 months (95%CI 7.2-24.0 months). OS data is not mature and does not reach the progress of the median CNS. Most of the treatment of related adverse events (Trams) is not serious and there are no deaths.
The results of this study believe that peripheral blood testing can be used as another biomarker detection method that guides ROS1+NSCLC clinical decisions. For this part of the patient, the efficacy of Ensteinib is consistent with the patients who use the organizational biopsy and have not observed new security incidents.
2
His Rebinib the treatment of ROS1+NSCLC Phase II Trust research data update
Taletrectinib is a new generation of powerful CNS permeability and selective ROS1-TKI. The ongoing Trust Study (NCT04395677) is a multi -center, open label, single arm, II, one -arm, II, one -arm, II Period clinical research. At the 2022 ASCO conference, Professor Li Wei, a Department of Lung Department of Shanghai Pulmonary Hospital, reported the latest research data of the study. (Summary number: 8572)
Studies are randomly assigned to patients with ROS1+NSCLC to be randomly distributed to queues without TKI treatment or had previously treated Cizidinib therapy and used his Rentinini 600mg QD. The endpoints of research include ORR, DOR, Disease Control Rate (DCR), PFS, IC-ORR and security.
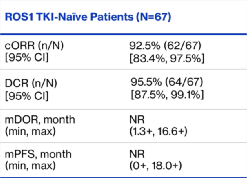
As of September 7, 2021, the ORR (CORR) confirmed in the queue that was not treated with TKI was 92.5%(95%CI: 83.4%-97.5%), and the DCR was 95.5%(95%CI: 87.5%- 99.1%).
In the queue that had been treated in the past, CORR was 50.0%(95%CI: 33.4%-66.6%), and DCR was 78.9%(95%CI: 62.7%-90.4%). Both queues have not reached MDOR and MPFS.
The most common Traes include diarrhea, nausea, vomiting, elevated transaminase, anemia, and decreased neutral granulocyte count, etc., as level 1 or 2; Essence

Among the patients who have not been treated with TKI and had previously treated Ketininib for ROS1+NSCLC, they all showed meaningful clinical effects and had good tolerance in the patient group. It is worth mentioning that he Rebinini showed clinical effects in ROS1 secondary G2032 mutant patients and brain metastases. Taletrectinib is well tolerated in the patient's group. 3
Cizibinib the treatment of EUCROSS research OS data update for ROS1 to re -discharge lung cancer
Eucross research is a European multi -center, single -arm, and phase II clinical study that explores the treatment of advanced ROS1 reinventing NSCLC. The previous research results found that the ORR of NSCLC patients for the treatment of ROS1 was 70%, and the median PFS was 19.4 months. (Summary number: 9078)
Studies are incorporated into the advanced/metastatic ROS1 rearracking NSCLC, and patients with stable brain metastases with stable diseases are treated with 250mg BID treatment of Kizininib. The main endpoints are INV-ORR (RECIST 1.1), and the secondary ending is PFS and OS.

After 55.9 months in the median follow-up, the main efficacy analysis set (PAS, n = 30) and the intention treatment group (ITT, N = 34) did not reach the median OS (95% CI, respectively 17.1-nr and 20.3- respectively NR). The study confirmed that OS was negatively correlated with brain metastases (P = 0.1805) and TP53 mutations (P = 0.015) and did not observe new security signals at the same time.
The updated OS data emphasizes the curative effect of Kizyinini for patients with ROS1 rearrangement NSCLC. At the same time, the results showed that patients with TP53 mutations or brain metastases were poor.

4
Ensteinib comparison of ROS1+NSCLC's phase III clinical research launch of ROS1+NSCLC
For ROS1 fusion positive NSCLC, brain metastases are common problems. However, the prognosis of patients with brain metastases lacks effective treatment plans. Both Endinininib and Klitininib are recommended for ROS1-TKI for ROS1 fusion-positive NSCLC first-line therapy, and they have performed well in terms of efficacy and tolerance. Cizinib's CNS activity may not reach the best standard, and Endotinib also has anti -tumor activity to intracranial lesions [PMIDS 30215676; 33646820)].
This is a comparison of Ensteinib and Cizidinib first -line therapy for local advanced/metastatic ROS1 fusion positive, accompanied or non -brain metastases of NSCLC patients, open labels, and multi -center III clinical research. (Summary number: TPS9141)
Studies are incorporated into patients with advanced/recurrence or metastasis patients who have not been treated in the past. Yes/no), randomly distributed to the Ensteinib 600mg QD or Kazidinib 250mg BID, until the disease progresses, unacceptable toxicity, exit research or death.

The main endpoint of the study is the BICR evaluation baseline CNS to transfer the PFS of NSCLC patients; the secondary end point is Orr, the CNS-PFS evaluated by BICR, the OS of the DOR and PFS, CNS and ITT people evaluated by BICR and researchers. At the same time, the quality of life, typical symptoms, and safety of the ITT crowd will be evaluated. This research is in progress.
references:
[1] EFFICACY/SAFETY of EntrestInib in Patients (PTS) Withros1-POSITIVE (ROS1+) Advanced/Metastative NSCLC from the Blood FIRSTAY Screen TRIAL (bfast).
[2] The Efficacy and Safety of Taletrectinib in Patients with TKI-Naïve or Crizotinib-Pretreated Ros1-POSITIVE NON-SMALL LUNG CANCER (NSCLC), 2022 ASCO, 8572.
[3] Crizotinib Inros1-RearRANGED LUNG CANCER (EUCROSS): Updated Overall Survival. 2022 ASCO, 9078.
Medical sources provide scientific information, which does not represent the point of view of this platform


- END -
23 cases of confirmed cases in Beijing on June 24, and 3 cases of input confirmed cases, 7 cases of asymptomatic infected infection were cured and discharged hospitalized.
From 0:00 to 24:00 on June 24, two new cases of confirmed cases were added, and there were no new suspected cases and asymptomatic infections; 3 newly added overseas input confirmed cases and 7 asympt
Gulang County organizational and livestock common disease prevention and control technology training course

In order to further strengthen the prevention and control of human and animal dise...