Choose the best in, cure the expected | Phase III lung cancer synchronous distribution chemotherapy/sequence chemotherapy, immunotherapy strong attack
Author:Cancer Channel of the Medical Time:2022.08.24
*For medical professionals for reading reference
Schurge Meticulum brought more hope to patients with NSCLC in the stage of III.
Phase III non -small cell lung cancer (NSCLC) is also known as local advanced NSCLC. It is a set of diseases with strong heterogeneity and poor prognosis. About 30%to 40%of lung cancer patients in my country are in the late stage of the first diagnosis [ 1]. With the continuous deepening of drug development of programming death receptor 1 (PD-1)/procedural death ligand 1 (PD-L1), immunotherapy has completely changed the phase III NSCLC treatment structure. Based on the results of Gemstone-301 research [2], Schurli Mutteria has been approved by the domestic synchronization or sequential chemotherapy in China. , Broaden -phase III cannot be removed from NSCLC immunotherapy.
On July 19, 2022, at the "Immunium Academy -20122 Lung -Cancer Immunotherapy MDT Seminar Seminar" hosted by Pfizer, the First Hospital of Jilin University shared two phase III NSCLC case immunotherapy. The "medical community" invited Dr. Zhang Yuyu, the first hospital of Jilin University, Dr. Zhang Yuyu, the first hospital of the case.
Share the pathogenesis:
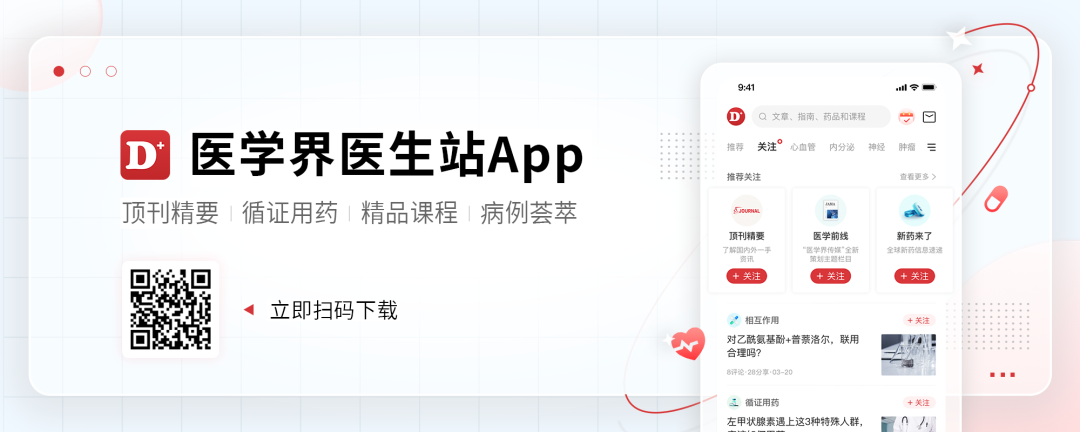
Patients, women, 48 years old. On March 24, 2020, the "left lung occupation" was discovered for "physical examination". PET-CT shows: high metabolic! 4 × 3.3cm in size, SUV: 13.5; left pulmonary and septum (4L/5/6 groups) lymph nodes high metabolism, size 2.3 × 2.1cm, SUV: 9.6. Psychological pathogenic prosthesis shows: (upper left lobe) lung adenocarcinoma. Gene test shows that EGFR, ALK and ROS1 are negative. Clinical diagnosis: left lung adenocarcinoma (CT2N2M0, IIIA stage).
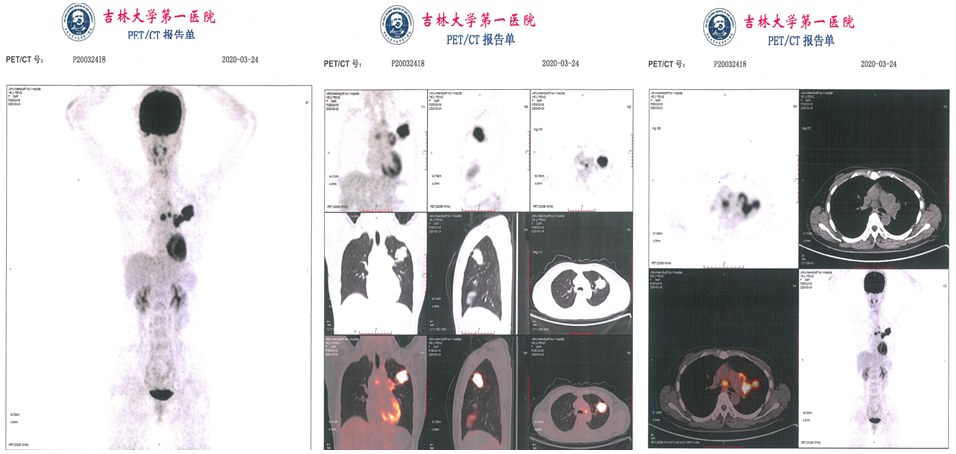
Figure 1. PET-CT examination results
▎ Synchronous chemotherapy
From April 2, 2020-May 24, 2020, the radiotherapy department is synchronized with chemotherapy, and the left lung tumor and hypertrophy metastasize the lymphatic treatment, DT: 60GY/30F/42D; +Cisplatin scheme chemotherapy. The efficacy assessment is part of the relief (PR).
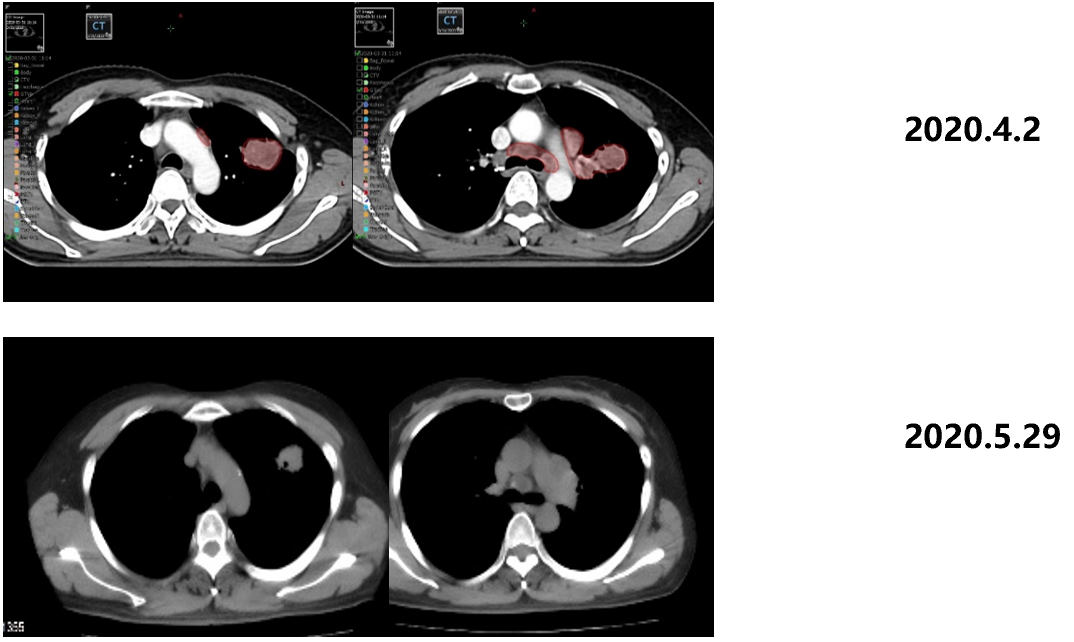
Figure 2. Chemotherapy results before and after chemotherapy
▎ Immunotherapy
From June 1st, 2020th to February 14, 2022, the patient was admitted to Gemstone-301 research to receive Schogly Mutteria immunotherapy. On September 27, 2020, 4 months after the chemotherapy, the immunotherapy was 6 cycles, and the curative effect was evaluated as PR. On January 14, 2021, 8 months after the chemotherapy, the immunotherapy was 12 cycles, and the efficacy was evaluated as PR.
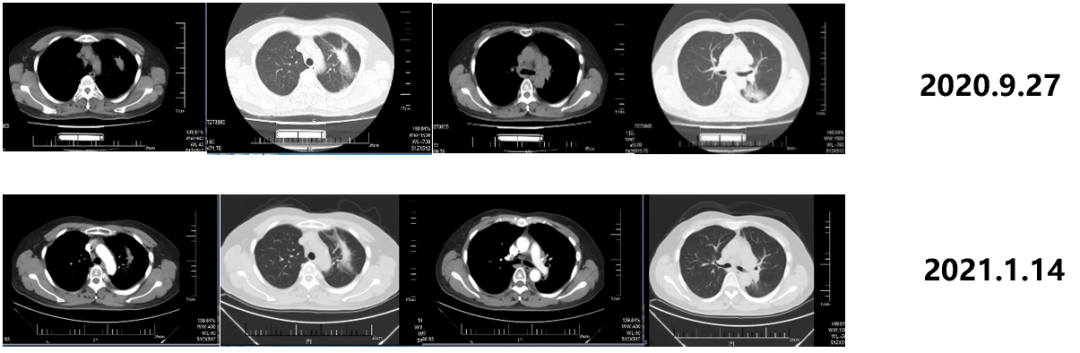
Figure 3. Imaging review results
On December 23, 2021, 20 months after the chemotherapy, the curative effect was evaluated as the stability of the disease (SD). On June 16, 2022, 26 months after the chemotherapy, the curative effect was SD.

Figure 4. Imaging review results
Two points of the disease:
Patient, male, 64 years old. On December 20, 2019, patients were visited for "discovery of lungs" due to physical examination. CT CT: The back of the left tip of the left lung occupies a position, the size is 4.6X5.1cm, and the bouring and the left pulmonary swelling lymph nodes are seen. Cervical ultrasound: A enlightenment on the left collarbone, 1.6 × 1.2cm, and the limits of the leather margin are unclear. Lymph nodes on the left side of the clavicle: lung adenocarcinoma. Gene detection shows that EGFR, ALK and ROS1 are negative. Clinical diagnosis: left lung adenocarcinoma (CT3N3M0, stage IIIC).
▎ Preface to radiotherapy
From January 11, 2020 to April 3rd, 2020, the 4th course of the Cancer Department of Peime Qucei+Cisplatin Plan Chemotherapy, the curative effect is SD.
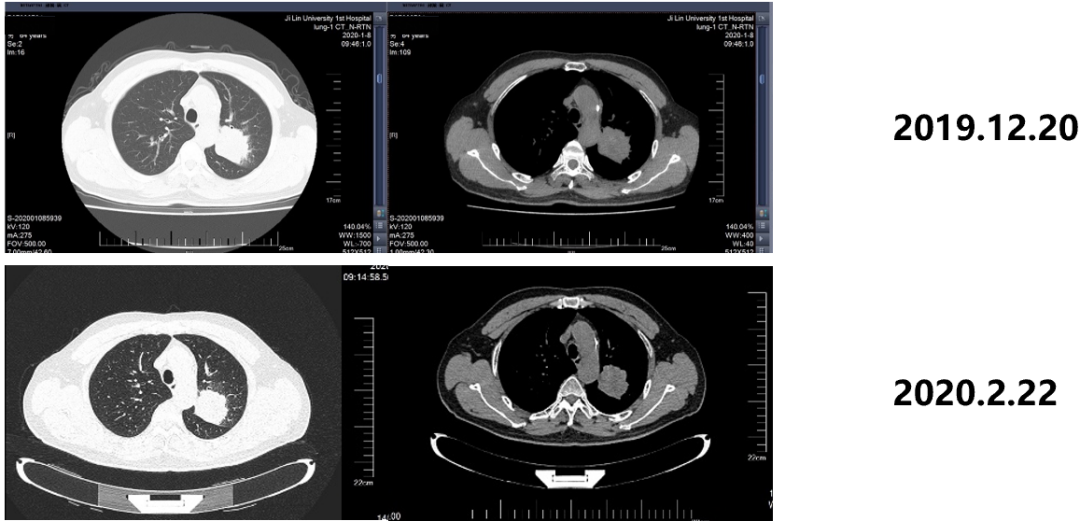
Figure 5. Examination results before and after chemotherapy
From April 13th, 2020-May 26, 2020, the lung lesions and pulmonary vertical diaphragm of the radiotherapy department are accumulated in the wild release of the lymph, DT: 60GY/30F/42D, and the efficacy evaluation is SD.

Figure 6. Test results before and after radiotherapy
▎ Immunotherapy
From June 10, 2020 to April 11, 2022, the patient was put into the Gemstone-301 study to receive Schogly Midumor immunotherapy. On August 20, 2020, 3 months after radiotherapy, the efficacy assessment was PR. On March 26, 2021, 10 months after radiotherapy, the curative effect was evaluated as SD. On November 9, 2021, 18 months after radiotherapy, the efficacy assessment was SD.
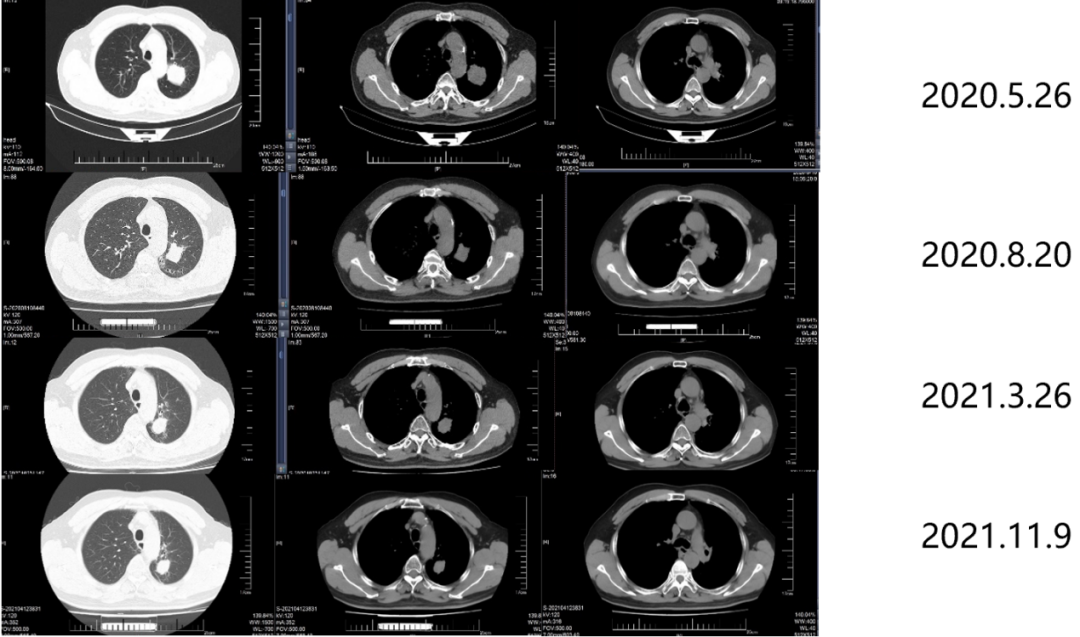
Figure 7. Imaging review results
On June 16, 2022, 25 months after the chemotherapy, the efficacy evaluation was SD.
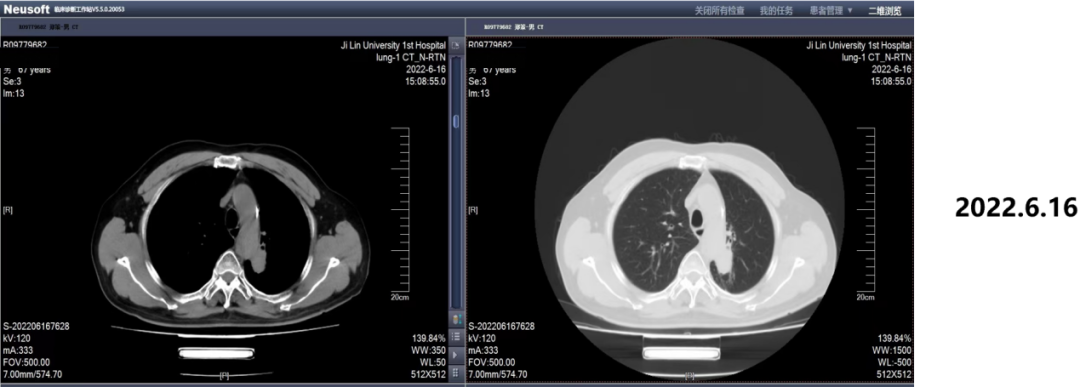
Figure 8. Image review results
Table 1. Tumor logo test results

Expert Reviews
After the above two patients, after synchronizing chemotherapy or sequential chemotherapy, Xingshutley Midticure treatment has received long -term benefits. Professor Dong Lihua conducted in -depth analysis and discussion of the two patients' diagnosis and treatment.
Schurge Metico -State Phase III applicable certificate filling phase III NSCLC immunotherapy blank
NSCLC accounts for about 85%of the total number of global lung cancer. When diagnosed with NSCLC, nearly one -third of patients are local advanced NSCLC. Most patients have lost their best surgical treatment opportunities when diagnosis, and their standardized treatment measures are synchronized chemotherapy/sequential treatment of chemotherapy [3]. Prior to the approval of the Phase III lung cancer indicator of the Schurge Micometer, there was no effective consolidation treatment for patients with Phase III NSCLC patients that did not occur after sequential chemotherapy. As the world's first PD-L1 monoclonal anti-anti-immune consolidation treatment after synchronous and sequential chemotherapy after the stage III cannot be cut in NSCLC. , Bring new hope for patient treatment. Professor Dong Lihua pointed out that the research results of the registered clinical research of Phase III NSCLC in the treatment of Schogly Midtopenia Treatment of Gemstone-301 [2] have been published in the top international tumor journal- "Liuye Knife-Oncology" and obtained it Comments from top experts in the global pharmaceutical academic field. Gemstone-301 is a stage II-stage study with random double-blindness and placebo control. It aims to evaluate Schogly Mippitive as a non-removed III NSCLC patient who does not develop after synchronization or sequential chemotherapy. Effectiveness and safety in China. A total of 381 patients from 50 centers in China were included in the study. For 14.3 months in the median follow -up of the Schutlist Single Anti -Anti -Group, when the placement group was followed by 13.7 months, the Schogly Midtop Party and the placebo group were blind. The Media Independent Center Examination Committee (BICR) evaluated the median no progressive survival period (PFS) was 9.0 months and 5.8 months, respectively. Schogly's anti -solitary anti 0.48-0.85, P = 0.0026), the 12-month PFS ratio of the Shusali Midumi and placebo group was 45.4% VS 25.6%.
Figure 9. PFS of the Schogly Midtop Party and the placebo group
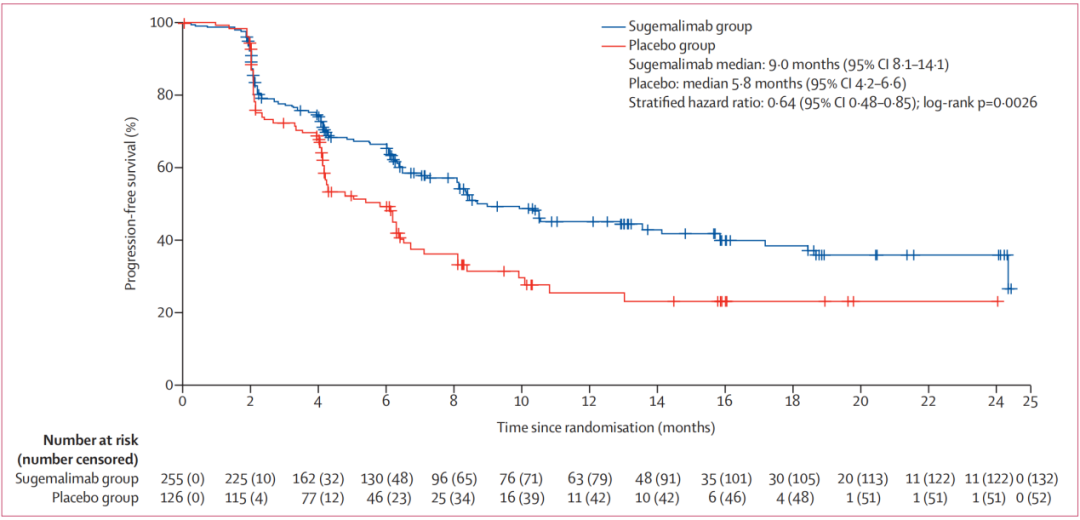
Gemstone-301 studied the dilemma of sequential chemotherapy patients, and officially clarified that the phase III non-removal NSCLC patients could benefit from receiving immunotherapy after synchronous or sequential chemotherapy.
Gemstone-301 research data update, once again affirmed the treated custody therapeutic position
In 2022, the World Lung-Cancer Conference (WCLC) announced the final PFS analysis results of Gemstone-301 research [4]. As of March 1, 2022, 62 cases (24.3%) and 26 cases (24.3%) and placeot groups (26 cases (24.3%) and placement groups (24.3%) and 26 consolation groups ( 20.6%) Patients are being treated. The medium follow -up time of the Schurli Meticuke and the placebo group is 27.1 months and 23.5 months, respectively. The 36 -month PFS rates were 38.6% vs 23.1% and 26.1% VS 0, respectively. For patients with sequential chemotherapy, the median PFS of the Schogly Micometer group and the placebo group was 8.1 months and 4.1 months (HR: 0.57). For patients receiving synchronous chemotherapy, the median PFS of the Schogly Midtopo and the placebo group was 15.7 months and 8.3 months (HR: 0.71).
In terms of median total survival (OS), the median OS of the Schogly Midumi group and the placebo group is NR and 25.9 months (HR: 0.69). For patients with sequential chemotherapy, the median OS of the Schogly Micometer group and the placebo group was NR and 24.1 months (HR: 0.60). For patients receiving synchronous chemotherapy, the median OS of the Schogly Midtopo and the placebo group was NR and 32.4 months (HR: 0.75). In terms of security, the proportion of the Schurge Metico -Summer and placebo groups of ≥3 treatment related adverse events (TRAE) were 11.4%and 5.6%, respectively, and no new security signals were found.
Professor Dong Lihua said that in general, compared with the placebo group, the PFS of the Schogly Midtopo group continued to benefit and good security. Preliminary data showed that the Schogly Midtopo group had the OS benefit trend.
Disclaimer: The case sharing and discussion involved in this article is only for education and communication, and does not involve consultation and intervention diagnosis and treatment. Relevant diagnosis and treatment decide by the doctor independently based on the patient's situation.
Choose Jiemei Product Manual

references:
[1] Gao Ming, Zhou Qing, Wu Yilong. In the immune era, the transformation of non-small cell lung cancer treatment in the immune era [J]. Evidence-based medicine, 2022,22 (1): 1-3,29.
[2] Qing Zhou, Ming Chen, Ou Jiang, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-black, multicentre, pHASE 3 trial. Lancet oncol. 2022; 23 (2): 209-219. [3] Yan Lanfang, Wuyun. Local advanced non-small cell lung cancer treatment status and outlook [J]. Progress of cancer, 2021,19 (19): 1957-1960.
[4] Y-l. Wu, q. zhou, m. Chen, et al. Sugemalimab vs placebo after or scrt in pTS with unresectable stage III nsclc: Final PFS Analysis of a Phase 3 Study.
Expert Introduction
Dr. Zhang Yuyu
First Hospital of Jilin University

Indications, doctorate in medicine, doctoral clinical medical post doctors
Visiting Scholar of the Institute of Cancer of Rogers University in the United States
Member of the Tumor Nutritional Support Group of the Chinese Medical Association Radial Tumor Treatment Branch
Secretary -General of the Jilin Provincial Research Hospital Society of Radiology Therapy Professional Committee
Member of the Northern China Tumor Radiation Treatment Cooperation Group
Member of the Radiovonant Youth Committee of Tumor Radiation Treatment Alliance
Member of the Beijing Medical Awards Foundation
Member of the Radible and therapy Commission of the Beijing Cancer Prevention Research Association
Member of the Professional Committee of the Beijing Science and Technology Medical Development Foundation
Cancer Branch of the Jilin Provincial Nutritionist Association
Vice Leadership Leader
2019 CSCO 35Under35 Most Potential Tumor Doctor
Presided one of the top -funded topics and 5 provincial scientific research topics. The first author published 7 SCI papers
Expert Introduction
Professor Dong Lihua
First Hospital of Jilin University

Director of radiotherapy
Professor and doctoral supervisor
Radial tumor treatment person in charge of Jilin Province Key Laboratory
The leader of the "Fourteenth Five -Year Plan" Medical Key Division of Changchun City
Member of the Chinese Medical Association Radiation Tumor Treatment Branch
Member of the Chinese Physician Association Radiation Tumor Therapist Committee
Member of the China Anti -Cancer Association Tumor Radiation Treatment Professional Committee
In the past 5 years, he has published SCI papers and schedules with the first author or responsible author. More than 10 items, including 2 special research and development specializations, 2 invention patents, and 2 provincial and ministerial awards.
It has been committed to the clinical, scientific research, and teaching work of tumor radiation therapy. The research direction is the clinical and basic research of the precise radiation therapy of the chest malignant tumor and the comprehensive treatment of tumor.
Approval code: PP-CEJ-CHN-0465 Validity: 2024-8-22
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
"Epidemic prevention and control+resumption of work and re -production" Xigu City Management made "combination boxing"

In the past few days, the Xigu District Urban Authority has based on his own job, ...
Which diseases are suitable for treatment?6 "old stubborn diseases" take you to understand the winter disease and summer treatment
What are the best diseases for San Futian as the best time for winter diseases and...