New idea of prostate cancer treatment: use Protac to lower therogens receptor!
Author:Yaizhi.com Time:2022.08.25
New idea of prostate cancer treatment: use Protac to lower therogens receptor!
Source: Yaozhi.com/Fei Xiang
Recently, the genetic Tick of the Pharmaceutical Giants Roche and the Jiyu Trust Innovation Research Institute, a subsidiary of Jimin Cito Trust, bought an exclusive license agreement at a price of up to $ 650 million. Protein degradation (TPD) drug: "JMKX002992".
Through this license agreement, Roche has added a new drug for degradation agent for transcription protein AR in the field of prostate cancer. Last month, Roche stopped clinical trials 1 of AKT inhibitors iPatasertib's prostate cancer.
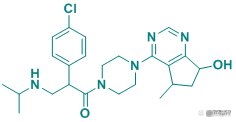
Figure 1 ipatasertib structure diagram. Picture source: I draw
Prostate cancer is an important cause of male cancer death. According to WHO statistics, 1.41 million first prostate cancer in the world in 2021, the incidence is second only to breast cancer, lung cancer, and colorectal cancer, ranking fourth, and has become the most common "male health killer".
Important targets of prostate cancer -A
Arogen receptor (AR) transcription factor is the main regulatory factor for the growth and survival of prostate cancer cells in normal glands and the growth and survival of prostate cancer cells. Therefore, AR targeted therapy can effectively improve the overall survival rate of patients with advanced prostate cancer who cannot be cured by surgery or radiotherapy.
Arogen receptor AR belongs to a transcription protein, and the disorder of transcription factors is related to a variety of diseases. And P53, MyC, NF-KAPPAB and other proteins, such as the male and other proteins, the male, and the male, the male, has always been the main target of prostate cancer. Many companies have developed targeted AR drugs.

Figure 2: The genetic expression of dependencies and does not depend onrogene. A ,rogen; AR ,rogen receptor; HSP, hot shock protein; ARV, androgen receptor variants. Picture source: Professor Wang Shaomeng's paper review
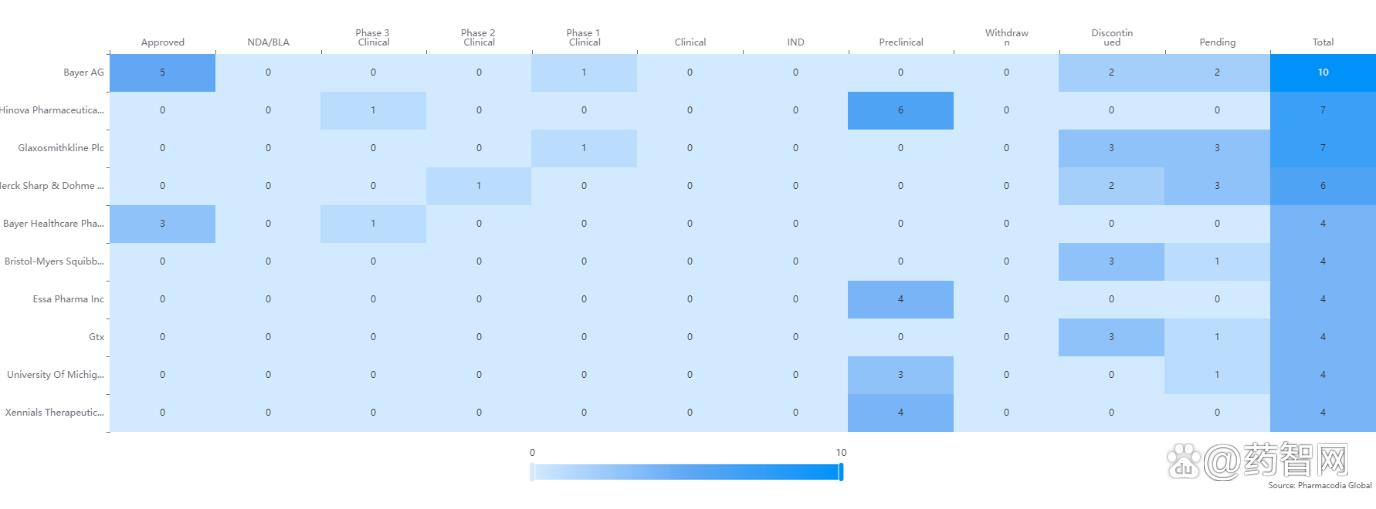
Figure 3 Drug sales targeting AR have risen in recent years. Picture source: Pharmacodia Global DataBase
Table 1: Related drug targets and related diseases including transcription factor protein, including AR.
Photo source: Literature finishing
The traditions on the market for AR for AR include Yang Sen's AR inhibitors Erleada (Apalutamide), Pfizer's AR inhibitor Xtandi (Enzalutamide), and NubeQA (Darolutamide) in Bayer.
Table 2 Top Ten Pharmaceutical Factory Observing AR Pharmaceutical Factory

Picture source: Pharmacodia Global DataBase
However, these proteins are traditionally considered a challenging drug target. In addition, the resistance of traditional inhibitors has hindered the clinical application of these drugs.
The protein degradation represented by Protac in the past few years has shown huge potential. They have the ability to overcome drug resistance and target the protein that were not previously used.
A variety of AR-PROTAC drugs have entered the clinical stage
In 2001, the Deshaies Laboratory of California Institute of Technology and the Cruise Laboratory of Yale University published their pioneering papers, officially recorded the concept of protein hydrolyzed targeting chimeric (Protacs). After 20 years, Protac, which targets AR, enters clinical trials.
ARV-10 (Bavdegalutamide) is the fastest Protac candidate drug for ARVinaS to degrade AR proteins to treat prostate cancer. It is currently in the clinical phase 2 stage. Avinas also has a oral proterac drug ARV-766 is conducting clinical trials. The compound structure of the ARV-766 that enters the first phase of the clinic is not yet public, but it is easy to be confused with the BDR4 degrade agent published by Professor CREWS in 2016. According to Avinas's official website, they are disclosed that they are developing ARV-766, which is a oral protrin degradation for AR for the treatment of men with MCRPC.
In preclinical study, ARV-766 degraded all AR resistance point mutations, including L702H, which is a mutation related to the treatment of Aibi Dragon and other targeted AR pathogenesis, and ARV110 (Bavdegalutamide) is clinical. There is no degradation in previous studies.
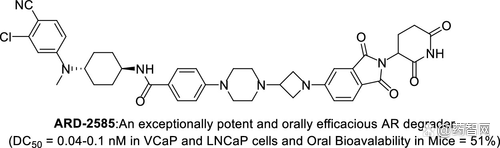
Figure 4 ARV-110 molecular chemical structure. Picture source: I draw
It is worth mentioning that the ARV-766, which is the same name as the AR degradant agent, is actually a negative control compound of the BRD4 degradant ARV771. BRD4 inhibitors ARV-771 observed some toxic side effects during the research and development process. Rain and others observe various toxic and side effects when using ARV-771 (drugs No. 76), including the skin discoloration of the skin in the injection site. Although the skin color will be restored after 2-3 days of medication. In addition, the even more worrying impact is the dose that mice cannot tolerate daily administration, and have to intermittently administers the day every day. It also observes the decline in mouse activity levels and spinal deformity. These related toxic mechanisms are unclear, but it may not be due to the unique side effects of Protac. Because in the RNAI mouse model, the suppression of BRD4 has proven to cause reversible epidermal hyperplasia and hair loss, but in general, Protac's income risk ratio is still good. Figure 5 ARV-771 molecular chemical structure. Picture source: Literature report

Figure 6 "ARV-766" in the 2016 CREWS paper
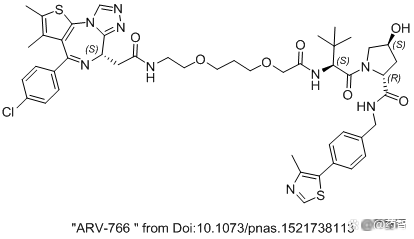
Note: The result should not be the real ARV-766 structure in the first phase of the first phase. The structure in the article is Protac based on BRD4 inhibitors JQ1. It was originally a article from Professor CREWS (10.1073/PNAS.1521738113) in 2016. This compound in the paper is not activated. Because the E3 is connected to VHL, VHL's cosmetic heterogeneous body will lead to whether the activity is, especially suitable for Protac negative control objects. CIS-HYDROXYPROLINES cannot combine E3 Ligase. Trans-Hyp can combine E3 Ligase. At present, the clinical ARV766 structure is still uncertain and may be reused. The structure in the figure may be the structure of the BRD4 degradant in the old text.
Recently, the academic community has also made breakthroughs in the development of degradants for targeted AR:
In September 2021, Professor Wang Shaomeng of the University of Michigan's Drug Chemistry reported on the ARD-2585 oral decomposure of Protac. This compound was aimed at degradable AR design. With excellent protein degradation activity, the DC50 is less than 0.1 nm. The IC50 values in the two cell lines related to prostate cancer are 1.5 nm and 16.2 nm in the IC50 in VCAP and LNCAP, respectively.
In addition, in mouse experiments, it shows 51%of oral biological utilization, and is better than the growth of the tumor model than the Enzalutamide, and has not observed toxicity.
Figure 7: ARD-2585 structural formula and active data. Picture source: jmc thesis

In 2022, Professor Wang Shaomeng introduced the journey of developing AR degrade agent development in the pharmaceutical industry in JMC. In the early days, scientists of Japanese Takeda used IAP ligands to design dual -function degradation agents. Snipers dependent protein dependent protein. Compound 132 is such a Sniper molecule. VCAP cells inhibit activity IC50 value reached 1 μm.
Compound 135 to 137 comes from the team of Professor CREWS, the founder of Protac, and achieved lower -level degradation effects below Nonorm's order -level degradation in VCAP, LNCAP, 22RV1 and other prostate cancer -related cell experiments. However, although compounds 136 and 137 have excellent degradation capabilities, their oral biological utilization in animals is low, which limits their further development.
ARV110 is a major adult of AR degrading agent. It is connected by a strong inhibitor. The CRBN ligand content is connected through rigid connection. It has excellent AR degradation effect, prostate cancer cells inhibitory activity, good oral medicine properties and in Animal experiments show anti -tumor effects.
Figure 8: The chemical structure of the Protac targeting AR. Picture source: JMC Thesis review

Summarize
In the past half a century, pharmaceutical companies and academic research laboratories have invested a lot of energy to develop androgen receptor drugs, including the Astronomical Removers and inhibitors that have been approved by FDA. The second -generation AR inhibitors Enzalutamide, Apalutamide and Darolutamide are clinically selective, powerful, effective and less side effects.
However, the rapid appearance of these drugs to resist the development strategies for new targeted androgen receptor drugs. Different from traditional small molecular drugs, targeted protein degradation (TPD) drugs represented by molecular gum and Protac are the side effects of reducing traditional small molecular drugs due to off targets, and can also overcome the increase and mutation of inhibitors' drugs to increase and mutate by target protein expression. Caused by drug resistance, as well as the characteristics of catalysts, can be repeatedly used. 1 TPD molecule can repeatedly degrade the pathogenic target protein.
However, the Protac compounds are currently facing the problem of excessive molecular weight and poor patent properties. This means that oral administration needs to increase dosage, causing potential toxic and side effects. In addition, not all proteins can be degraded efficiently. For example, BRD4 has potential on target side effects, and Irak4 inhibitors and degradants encounter some difficulties. Although there are currently no drugs approved by FDA, after more than 20 years of technical accumulation, Protac will eventually usher in its era. References: 1.https://data.pharmacodiaglobal.com/2.https: //proteovant.com/about/3.arvinas drug pipeline https://www.arvinas.com/pipeline-proPeeline4. Pharmaceutical targeted AR pharmaceutical pipeline https://en.kintor.com.cn/intro/30.html5.http://www.biospectator.com/view/news_View.phratcid=17005 6. Treatment strategy of hormone receptors: https://pubs.acs.org/doi/10.1021/acs.jmedchem.2c007167. The paper review target transcription protein drug development Outlook: https://pubs.acs.org/doi/pdf /10.1021/acs.jmedchem.2c006918. The paper reviews the propac connection sub -design: https://www.explorationppub.com/uploads/article/a100218/100218.pdf9.vhl affects the paper. .org/Doi/Full/10.1021/JACS.8B05807
- END -
Professor Chen Shaohong of our school was selected as "Master of Chinese Medicine", Professor Ai Ruyi and Professor Xiong Dajing were selected as "National Famous Chinese Medicine"

On July 20, the Ministry of Human Resources and Social Security, the National Heal...
Ruijin Hospital Mi Jianqing and Xu Jieyou talked about CAR-T diagnosis and treatment experience

With the aging population in China, the incidence of multi -bone marrowoma (MM) in...