2022 WCLC 丨 NSCLC KRAS Huazhang sounds, four studies and one article speed view
Author:Cancer Channel of the Medical Time:2022.08.27
*For medical professionals for reading reference
What are the wonderful studies of 2022 WCLC?
The International Cancer Research Association (IASLC) World Lung Cancer Conference (WCLC), as the world's largest lung cancer and other chest malignant tumor academic conferences, has received widespread attention from global professionals every year. The editor sorted out the research progress of targeted therapy for targeted therapy for non-small cell lung cancer (NSCLC) at the WCLC conference in 2022 [1-4] for reference.
Sotorasib combined with SHP2 inhibitors in the application of KRAS P.G12C mutations NSCLC and other physical tumors
(Summary number: OA03.03)
Sotorasib is a specific, irreversible KRAS G12C inhibitor. In the CodeBreak100 I/II test, the genome changes of receptor tyrosine (RTK) are considered to be a common mechanism for SotorAsib to resist. In animal experiments, SotoraSib and SHP2 inhibitor combined with RTK signal conduction that can damage RAS to enhance the efficacy of anti -tumor.
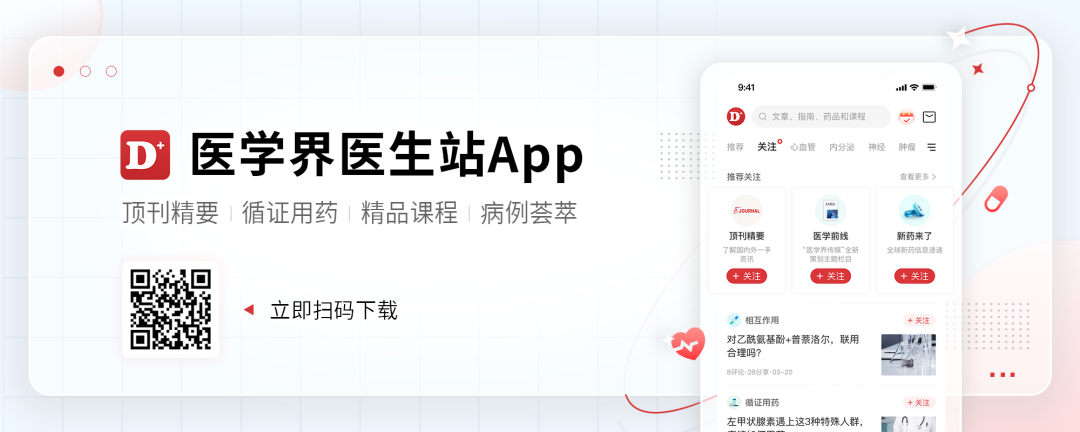
A study reported the safety and efficacy data of SotorASIB combined with RMC-4630 (a small molecular SHP2 inhibitor) to treat KRAS P.G12C mutations NSCLC and other physical tumors. In 2022, the WCLC conference announced the efficacy results of patients with NSCLC. As of April 11, 2022, 11 patients with NSCLC were included in the median number of previous treatment. G12C inhibitor treatment. Among the 11 patients with NSCLC, 3 (27%) were proven to have partial relief (PR), and the objective relief rate (ORR) was 27%, of which 2 of them continued when the data deadline was dead; 7 cases reached disease control. DCR (disease control rate) reaches 64%. Among the six patients who had not received KRAS G12C inhibitors in the past, ORR was 50%and DCR was 100%.
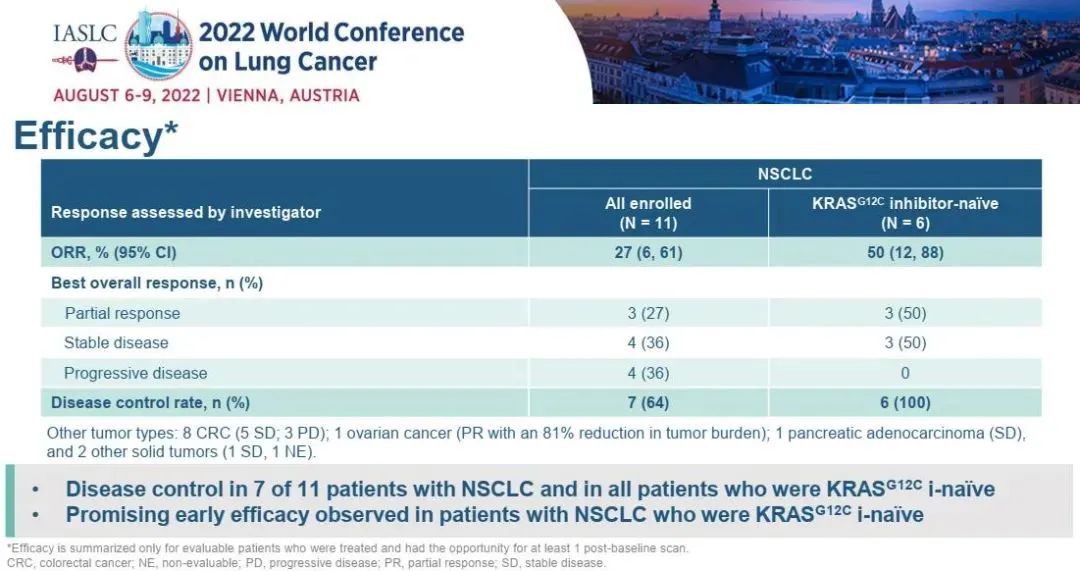
Figure 1. SotoraSib combined with RMC-4630 to treat NSCLC's efficacy
Of the 4 patients with the highest dose of 2 doses of RMC-4630 combined with SOTORASIB, of the patients with first treatment of NSCLC in the first treatment of NSCLC, 3 cases (75%) reached proven PR and 4 (100%) disease control.
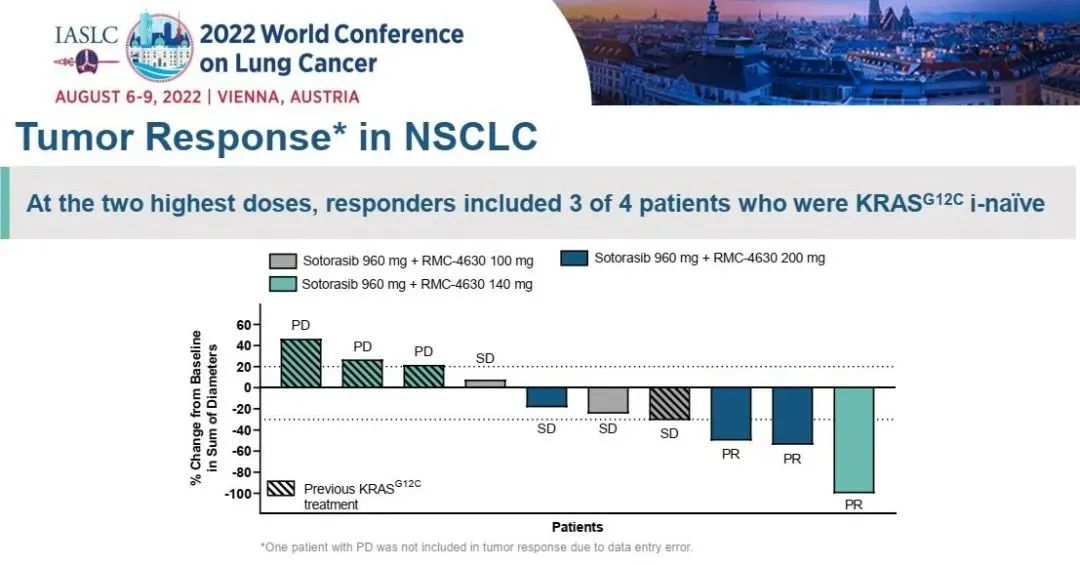
Figure 2. Tumor retreat in patients with NSCLC
In terms of security, SotoraSib combined with RMC-4630 treatment is safe and tolerant. There is no treatment related adverse reactions (TRE) during the completion of the dose. 63%of patients produce different degrees of Trae, including edema, diarrhea and dry mouth. SotoraSib combined with RMC-4630 observed promising clinical activity among KRAS P.G12C mutations, and benefits from patients who have not received KRASG12C inhibitors.
Evaluate the efficacy of GDC-6036 single medicine treatment KRAS G12C mutant NSCLC patients
(Summary number: OA03.04)
GDC-6036 is an oral, efficient, selective KRAS G12C inhibitor. A research on an open label, dose increase and dose expansion research. GDC-6036 single medicine or in patients with local advanced or metastatic physical tumors in KRAS G12C mutations or metastatic physical tumors Joint treatment, the WCLC conference announced the research data of NSCLC for GDC-6036 single medicine treatment.
As of May 13, 2022, a total of 135 patients in the study group were studied, of which 59 of them were NSCLC. In terms of security, in the treatment of NSCLC and other solid tumors, the common Trams of GDC-6036 is nausea, vomiting and diarrhea, mostly level 1, and 7 patients with 7 patients have nothing to do with drugs. In general, patients can manage adverse events by supporting treatment or dose adjustment. In terms of efficacy, for patients with KRAS G12C mutant NSCLC treated with GDC-6036, the unrefined ORR is 53%(30/57), and the confirmed ORR is 46%(26/57). The analysis of pharmacokinetics shows that the half-life of GDC-6036 once a day (QD) is consistent with GDC-6036 administration (50-400mg), with an average half-life of 13-17 hours. Patients with GDC-6036 (400mg QD) in dose extension are expected to reach the exposure of the largest targeted covalent in non-clinical research.

Figure 3. The orr of KRAS G12C mutant NSCLC patients
The study confirmed that GDC-6036 had good tolerance and controlled overall safety. The GDC-6036 single-drug treatment of the KRAS G12C mutant NSCLC, which was previously treated, showed encouraging anti-tumor activity. The pharmacokinetics supports GDC-6036 once a day, and can observe the combination of high KRAS G12C targets in NSCLC.
CODEBREAK 100/101 Research: SotorASIB combined with Paborzab or Aditizab's advanced KRAS P.G12C mutation NSCLC (Abstract number: OA03.06)
In 2022, the WCLC meeting reported the first research results of SotorASIB combined immunotherapy. A stage IB study called CodeBreak 100/101 will divide the KRAS G12C mutant NSCLC patients who have not been treated with KRAS G12C inhibitors into 12 queues in the past. Pearl Mipida treatment. Half of them are Lead-in, that is, the patient conducts a 21-day or 42-day SotoraSib single drug treatment before the first medication.
The study incorporated in 58 patients, with a median follow -up for 12.8 months. Of all the 12 queues, 17 patients with 17 patients reached confirmation and relieved, ORR was 29%, and DCR was 83%. Of the 17 patients with relief, the mid -position relieving duration (DOR) was 17.9 months.
Figure 4. SotorASIB combined with Paborzab or Aidarzumab treatment
The median total survival (OS) of SotorASIB combined immunotherapy is 15.7 months. In the Lead-in queue and the Concurrent queue, SotorASIB combined with Paborzab's ORR for 37%and 32%, respectively. The ORRs of Pearl Mipida are 20%, and SotorASIB combined with Paborzab's treatment has obtained more effective relief.
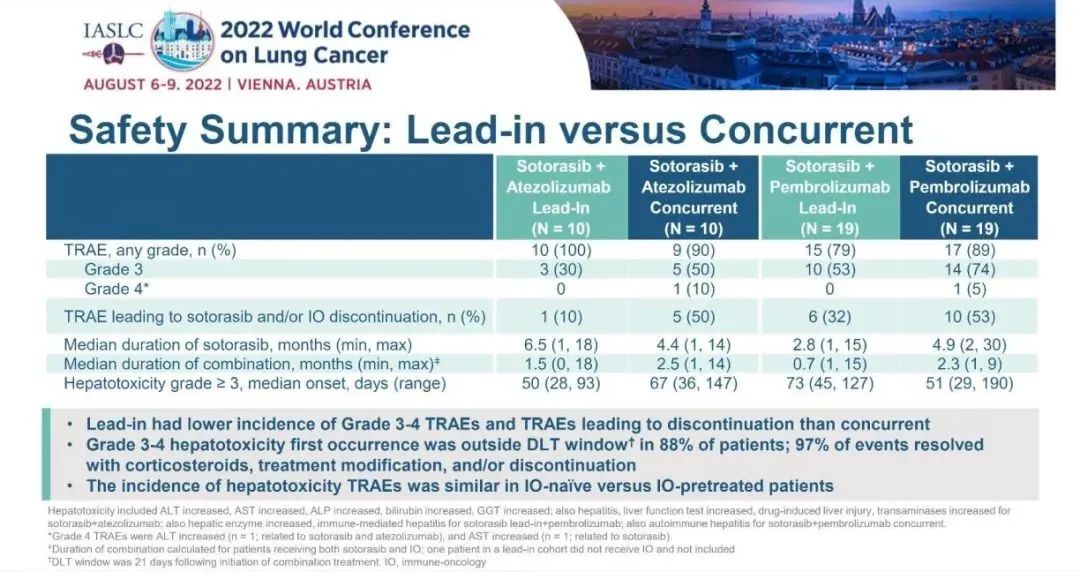
In terms of safety, the incidence of common 3-4 TRAE is 59%, mainly hepatic toxicity, which is manifested as the level of level of gifda transaminase (ALT) and croaramase (AST). The probability of SOTORASIB combined immunotherapy is higher than that of SotorASIB single drug therapy. Compared with the Concurrent queue, the Lead-in queue shows lasting clinical efficacy, and the incidence of 3-4 TRAE is low.
Figure 5. SotorASIB combined with Paborzab or Ayidzumab treatment safety
A phase I test: the safety and effectiveness of D-1553 in KRAS G12C mutations in NSCLC patients
(Summary number: OA03.07)
The D-1553 is a biochemical inhibitor of the KRAS G12C. It can selectively and irreversible KRAS G12C mutant protein to make it in a non-active GDP binding state. A phase I, open labels, and multi-center studies for the advanced or metastatic NSCLC patients that have progressed after receiving standard treatment for the treatment of standard treatment, and conduct D-1553 safety, pharmacokinetics (PK) and efficacy assessment of D-1553.
As of May 9, 2022, a total of 79 patients were included in the study. The patient had received the number of neutral systemic treatment lines, of which 42 of them (53.2%) had previously received ≥2 treatment. At the deadline of the data on May 9, 2022, the median follow -up time was 21.7 weeks. Of all 79 patients, 53 patients (67.1%) were still being treated. 68 patients (86.1%) traded Trae, most of which were level 1-2. The most common (≥20%) Trae is AST elevation, elevation of ALT, elevated γ-glutamyl metastases, combined with bilirubin elevation and anemia. Unprocessed level 5 Trape.
73 patients with all dose levels can evaluate tumor reactions: 29 patients PR, 38 patients with stable disease (SD); ORR and DCR were 39.7%(29/73) and 91.8%(67/73), which did not reach reaches Mid -position DOR, but 25 patients (86.2%) of 29 patients were still under treatment, of which 14 patients were DOR ≥ 12 weeks. For no progressive survival (PFS), 57 (78.1%) subjects have not reached the incident. Studies have confirmed that D-1553 has good tolerance, and has good anti-tumor activity in the KRAS G12C mutant NSCLC that has been prepared in large quantities.
This material is supported by Astrikon, for medical professionals for reference
Approval number: CN-100805 Expired Date: 2023-8-15
references:
[1] Bob T. Li, Kristen a Marrone, Christine M Bestvina, et al. Sotorasib in Combining with RMC-4630, A shp2 inhibitor, in kas p.g12c-mutated nsclc and Other Solid Tumors. 202222222222222222222222222222222222222222222222222222222222222222222.
[4] Hong Jian, yiping zhang, zhengbo song, et al. Safety and effect of d-1553 in Patients with k12c multived non-Small Cell LUNG CANCER: A Phase 1 TRIAL. 2022WCLC.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
"Ecological Prince" in the desert

It is the northern meal. The nickname is 10,000 -year -old dirty. It can grow wild...
As the saying goes, the effect is poor?The key to the intervention of Chinese medicine, the key to work is not to eat rice

Open -columnAs the saying goes is the first Chinese medicine department with infla...