One article interpret
Author:Cancer Channel of the Medical Time:2022.08.27
*For medical professionals for reading reference
Impower133 and Caspian are studied and interpreted.
Small cell lung cancer (SCLC) is a subtype of lung cancer. It belongs to high -level neuro endocrine tumors. The degree of malignancy is high. Most of them are advanced during diagnosis and the survival rate is extremely low. According to the scope of the lesion, SCLC is divided into a limited period and extensive period. About two -thirds of the SCLC is in a wide range of periods during diagnosis; chemotherapy schemes based on platinum can temporarily alleviate the clinical symptoms of patients in a wide range of patients, but the recurrence rate Very high, the prognosis is generally worse [1]. With the rise of immunotherapy, a variety of immune drugs are widely used for the treatment of various types of cancer, and the curative effect is significant. At present, a number of studies have shown that immunotherapy has significant effects on patients with SCLC. Based on two large-scale IMPOWER133 [2] and Caspian research [3-5], the "China Clinical Oncology Society (CSCO) small cell lung cancer diagnosis and treatment guidelines (2022) "The first -line therapy for Adidarzumab and Du Riudi Mipida as a preferred recommendation for level Ⅰ is used as a preferred recommended for patients with SCLC [6]. This article interprets two studies.
IMPOWER133 Study:
Aidleyzumab combined with chemotherapy
IMPOWER133 research is a phase III clinical study that confirms PD-L1 combined with the first-line treatment of PD-L1 combined with chemotherapy. The study is a global multi -centered phase III study with random dual -blindness and placebo control. 403 patients with unprecedented periods of SCLC were included in 403 unprepared period, including stable brain metastases with stable treatment. Patients with admission groups are randomly 1: 1, and giving Adilizumab combined with Karplatin and Renposidon schemes and sequential Antidi Mipidu maintained treatment or placebo+card platinum combined with Polycin. Settles are treated until the disease progresses or adverse reactions may occur or no longer clinical benefits. The test group and control group chemotherapy schemes are 4 cycles [2].
After 13.9 months, the research results showed that [2], the median total survival (OS) therapy group of the Adelico Mummy was significantly extended for 2 months (12.3 months vs 10.3 months, P = 0.007), the 1 -year OS rate was also significantly higher than the chemotherapy group (51.7%vs 38.2%). No progressive survival period (PFS) is 5.2 months and 4.3 months, respectively (P = 0.02). The 6 -month PFS rate and 12 -month PFS rate are higher than the chemotherapy group (30.9%vs 22.4%; 12.6%vs 5.4% To. Based on the results of this research, the US Food and Drug Administration (FDA) in 2019 and the State Drug Administration (NMPA) in 2020 has approved the first line of the first line of Ayidozumab and pyrine for the extensive period SCLC treat. In 2022, the CSCO guide still recommend this solution as a level I treatment [6].
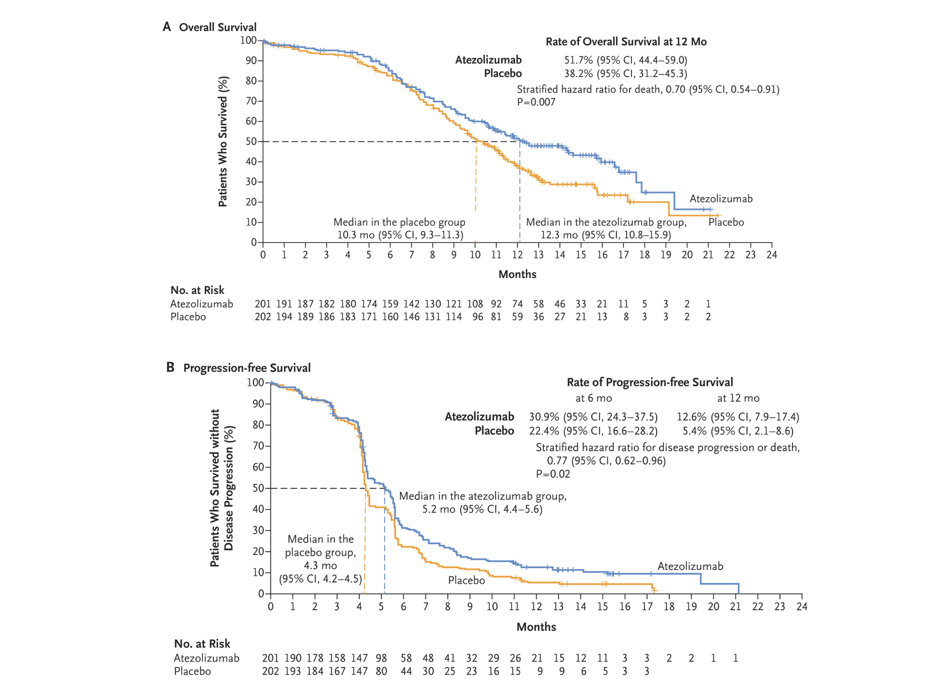
Figure 1. In the study
Caspian research:
Du Lilin Mipida Anti -combined chemotherapy
Caspian research is another phase III clinical study that confirms PD-L1 combined with chemotherapy first-line treatment. Lancet). This study is a phase III study with a random, open label, and a global multi -center. It aims to evaluate whether the combination of combined with the combination of Platinum+Renpacid chemotherapy when the first -line treatment of SCLC patients can be treated in a wide range of SCLC patients. Bringing survival benefits to patients. The study was included in 805 patients with a wide range of SCLC patients, including asymptomatic brain metastases and stable brain metastases of unsecured brain metastases. Among them, the plan of Du Diabuyu+Chemotherapy Group is the 4 cycles of Tuolimu 1500mg combined with pyrine+card platinum/cisplatin q3w. The progress of the disease or toxicity is not tolerated; the simplicity therapy group scheme is relied on Polycin+Card platinum/cisplatin Q3W, which can treat up to 6 cycles [3].
The results showed that [3], compared with the simple chemotherapy group, the median OS of the combined chemotherapy group of Durai can reach 13.0 months and reduce the risk of death by 27%(HR = 0.73, P = 0.0047); the two groups of the two groups The 12 -month OS rate and 18 months OS rates were 54%VS 40%and 34%VS 25%, respectively. Based on this significant benefit, the FDA approved the first -line treatment of patients with SCLC patients in the broader period of SCLC in 2020.
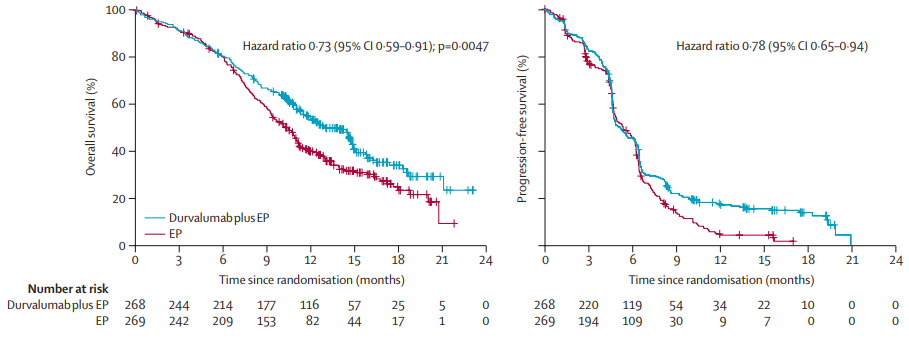
Figure 1. CASPIAN studies moderate Paeyu's OS and PFS both significantly improved [3]
It is worth mentioning that Caspian studied the 123 Chinese patients in the Chinese queue (limited to the combination of combined chemotherapy group and the simplicity chemotherapy group), compared with the simple chemotherapy group (10.9 months) The mid-position OS of the Youzab combined chemotherapy group reached 14.4 months (HR = 0.65, 95%CI 0.41-1.03) [4].
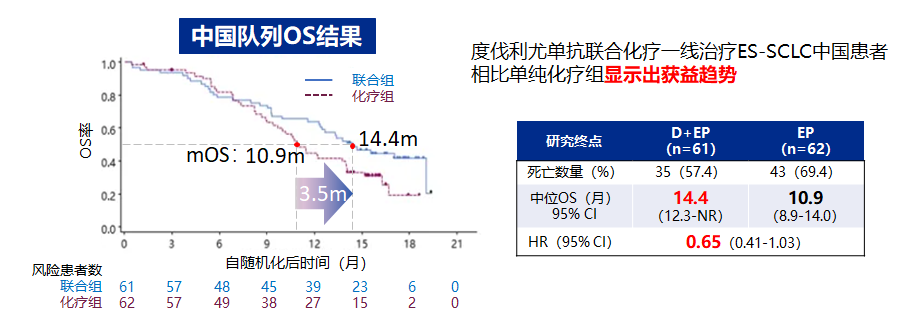
Figure 2. Caspian studies the Chinese queue medium Taeyu's OS OS benefit trend [4]
The 3 -year OS results of Caspian's research updated in 2021 showed that as of March 22, 2021, the median follow -up time was 39.4 months. Compared with the chemotherapy group, the risk of death at Durai Polytaba+chemotherapy group is 29%(HR = 0.71, 95%CI 0.60-0.86; P = 0.0003). The 3 -year OS rate was 17.6%VS 5.8%, showing the "long tail effect" of immunotherapy. Compared with the chemotherapy group, the long -term OS benefit advantage is obvious [5]. Based on the excellent performance of CASPIAN research, in 2021, NMPA approval of Permutab combined with the first -line treatment of patients with a wide range of SCLC. In 2022, the CSCO guide also revised the scheme to class Ⅰ recommendation [6].
Summarize:
Caspian research included in the patient's baseline is basically the same as the IMPOWER133 research, but it is slightly different in research and design. In the Caspian research, an unable to treat the asymptomatic brain metastases in the CASPIAN research. The chemotherapy scheme can choose cisplatin or card platinum, and the dosage interval after chemotherapy can receive up to 6 cycles of chemotherapy; in the end, Caspian research With significant results, the 3 -year OS rate reached 17.6%, which was nearly 3 times from the therapy group, and the long -term survival benefits were significant [5]. IMPOWER133 Studies allowed to be included in patients with stable brain metastases. After chemotherapy, the treatment group can be used to prevent preventive omnidirectional radiotherapy; chemotherapy schemes are karplatin; the control group receives 4 cycles of chemotherapy; in the end, OS is 12.3 months. 1 year OS rate is 51.7%[2]. Overall, in the extensive period of SCLC's first -line treatment, there are two types of immunotherapy approved in China. With the listing of Adidarzumizumab and Daguelu, it brings more Chinese SCLC patients in China Preferably treatment plan. In the future, we look forward to more and more immune drugs to create longer survival benefits for patients with SCLC.
references:
[1] Wu Wei. Immune examination point inhibitor in the application of small cell lung cancer [J]. Chinese medical innovation, 2022,19 (04): 183-188.
[2] Horn l, masfield as, szczęsna a, et al.first-line atezolizumab plus cheetherapy in extensive-stage small-cell lung car J med.2018 dec 6; 379: 2220-22: 220-22: 2220-22: 2220-22: 2220-22: 2220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 220-22: 2320-220-22: 220-22: 220-22: 220-22: 2220-220-22: 2229220
[3]Paz-Ares L,Dvorkin M,Chen Y,et al.Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer(CASPIAN):a randomised,controlled,open -label, Phase 3 trial.lance.2019 nov 23; 394 (10212): 1929-1939.
[4] NCBI-WWW Error Blockd Diagnostic. (N.D.). Retrieved August 26,2022, from https://clinicaltrials.gov/ct2/Show/nct03043872
[5]Paz-Ares L,Chen Y,Reinmuth N,et al.Durvalumab,with or without tremelimumab,plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer:3-year overall survival update from Caspian.esmo Open.2022 APR; 7 (2): 100408.
[6] Guide Working Committee of the China Clinical Oncology Society. The China Clinical Oncology Society (CSCO) Small cell lung cancer diagnosis and treatment guidelines 2022 [M]. Beijing: People's Health Publishing House, 2022.
Approval number: CN-101699 Expired Date 2024-8-26
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
Is the mosquito repellent supplies of the mother and baby chosen?Traditional Chinese medicine experts send Chinese herbal medicine sachets and mosquito repellent
The Yangtze River Daily Da Wuhan client July 13th news Tanghe banana, bamboo forest pine yin, and insects crying ... Recently, many netizens who chased the drama played the simple and elegant Song -st
The first domestic new crown oral medicine Azf's fixed film is put into production in Pingdingshan

/Azf's fixed film production ceremonyOn the morning of August 2nd, the production...