It was once used as a "poison" to withdraw from the market, and now it has been returned again
Author:Medical community Time:2022.06.19

Another example of old medicine freshman
Written article | Fang Jingyu
Source | "Medical Community" public account
In modern medicine, the story of "old medicine" is not uncommon. For example, "Star" antilida drug aspirin has also shouldered the effect of "relieving heat and analgesia" for more than a hundred years. Vitamin A acid, which is effective for acute leukemia, was originally came out of the image of acne.
In these "old medicines and freshmen", the starting point of medicalists' research is basically some drugs with rich clinical experience, validity and safety with reliable evidence.
This is the practice of medical research. For a drug that has been proven to be harmful and unhelpful in clinical practice, rare people can get courage to "rectify their names."
But there are "exceptions".
In June 2020, the Fenfluramine (FDA) of the US Food and Drug Administration (FDA) was approved to treat the Dravet syndrome of rare diseases.
Earlier, Fen Fllaming had been asked to withdraw the market by the FDA in September 1997 for inducing cardiac valve diseases in September 1997. The FDA's "fishing" this time also marked that Fen Fllaming ushered in a new start after decades of ups and downs.
"Unknown Small Wind" in the weight loss market
Since ancient times, people's pursuit of "thin" has never stopped. On the one hand, the aesthetics of most cultures believe that "thinness is beauty", and on the other hand, obesity is associated with many physical diseases.
By the 1950s, a "birth" of a weight loss pill made people realize that it was not difficult to lose weight.
In 1947, American doctor Henry M.Ray began to use stimulant AMPHETAMINE, also known as "Africa's life") and dextRoamPhetamine to treat obesity.
Under the premise of having diet control and exercise, obese people can reduce weight by about 14%for only 90 days. This result quickly sensation in the medical community. At that time, pyramia was a prescription drug that could be easily obtained. If you can obtain considerable weight loss by taking phenibine, it will undoubtedly have a earth -shaking impact on obesity treatment.
Smith, Klinefrench, the inventor of Pyranopenia, smell business opportunities. In 1947, Pyramia drugs were recommended by the American Medical Association and used to treat obesity. Schick began to publicize DEXEDRINE (rightblyzate) as "weight loss medicine" in the advertisement. This move allowed DexEdrine to sales in 1949 to $ 73 million, up 28%from the drug's weight loss before weight loss.

Schick's advertisement for Dexedrine was published in the 1950s
At that time, patented drugs had already passed the patent period, and many pharmaceutical companies began to produce such drugs in order to profit in the field of obesity.
Among them, REXAR's Obetrol was officially approved by FDA in 1960. The medicine once was known for its crazy marketing methods and high popularity. In an advertisement in 1970, Rexar used the current popular ski sports to promote the benefits of weight loss. By providing "doctor samples" and other methods, it attracted doctors to prescribe OBETROL prescriptions for patients.
Thanks to the propaganda, in the 1950s and 1970s, the number of prescriptions for Penaline drugs in the United States surged. A survey showed that during the period from 1970 to early 1971, 5%of American adults had been prescribed at least one phenobyne drug.

Rexar's advertisement for Obetrol, 1970
At the same time as thin, Americans paid a great price.
In the 1950s of the beginning of the prevailing of pyramia drugs, people had recognized the potential and dependence of abuse. Especially in a variety of diet pills, Methamphetamine, its common name is "methamphetamine", which has caused serious social problems globally.
It is generally believed that in 1970 alone, about 2.1 million people in the United States abusedzhenyline drugs, of which about 320,000 were addictive. The "Penalia Crisis" gave the US government as a start to drink. In 1970, the United States passed the Controlled Substances Act (CSA) to regulate the phenomenal drugs in the Appendix of the bill.
The good era of phenylphenyne drugs is gone.
"Daba" pushed, better than thousands of words
In the third year after the CSA, in 1973, the US FDA approved a new type of weight loss drug Fenflora, nominated for it "combined with diet restrictions and exercise, for treatment of obesity."
At the same time, more use in the US market is Phentermine approved in 1959.
But clinicians are not satisfied with Finte. Although the drug has a small adverse reaction and the control level is low, the weight loss effect is also greatly reduced compared to the traditional phenobyrine drugs (an average weight loss of 7%after 8 weeks). Many patients have not satisfactory effects after taking Fenteming, and they turn to other treatment methods such as weight loss surgery. Therefore, clinicians are trying to find Fentming's "substitutes" or to enhance the effect of Fentming's weight loss effect.
Fenfllaming and Fenter have similar structures and also belong to a pyramine. The pharmacological effects of the two are mainly to suppress appetite, the mechanism, effect, and side effects are also similar, the spiritual excitement has a weak effect, and the abuse potential is less. Appendix II) control, very small sales and use restrictions. Fen Fllaming was unknown in the weight loss pill market at first, mainly because it was too new. " In the "Kefauver Harris Amendment", the US government should "respond to the suspension of the incident" and the 20th century of the 20th century of the "important amendment to the Federal Food, Drug and Cosmetics Act in 1938") In the 1970s, doctors prescribed new medicines often carefully, so as not to "turn" because of adverse reactions. During the same period, Fentming's clinical experience and marketing were mature, and doctors were more willing to use their familiar drugs.
In 1984, due to a wonderful article, Fenfllaming began to usher in his own era.
This article was published by American doctor Michael Weintraub and others. The article states that the researchers conducted a well -designed placement control, cross, and double -blind clinical research. The results confirmed that the combination of Finflurara and Fentming could effectively reduce weight and avoid weight rebound after weight loss treatment.
After the study and publishing, the combination of Fen Fllaming and Fentming officially "debut" with the nickname "Fen-Phen", which has won the favor of many doctors and patients in the United States.
According to statistics, from 1992 to 1997, Fentming's prescription volume in the United States increased by 442%, and Fenflumin's prescription volume increased by 6390%. In 1996 alone, "Fen-Phen" was as high as 18 million in the United States.
Under the frenzy, Fen Fllaming's obscure drug has become the darling of the market. A.H. Robins, which produces Finflurajima, earn a lot of money.
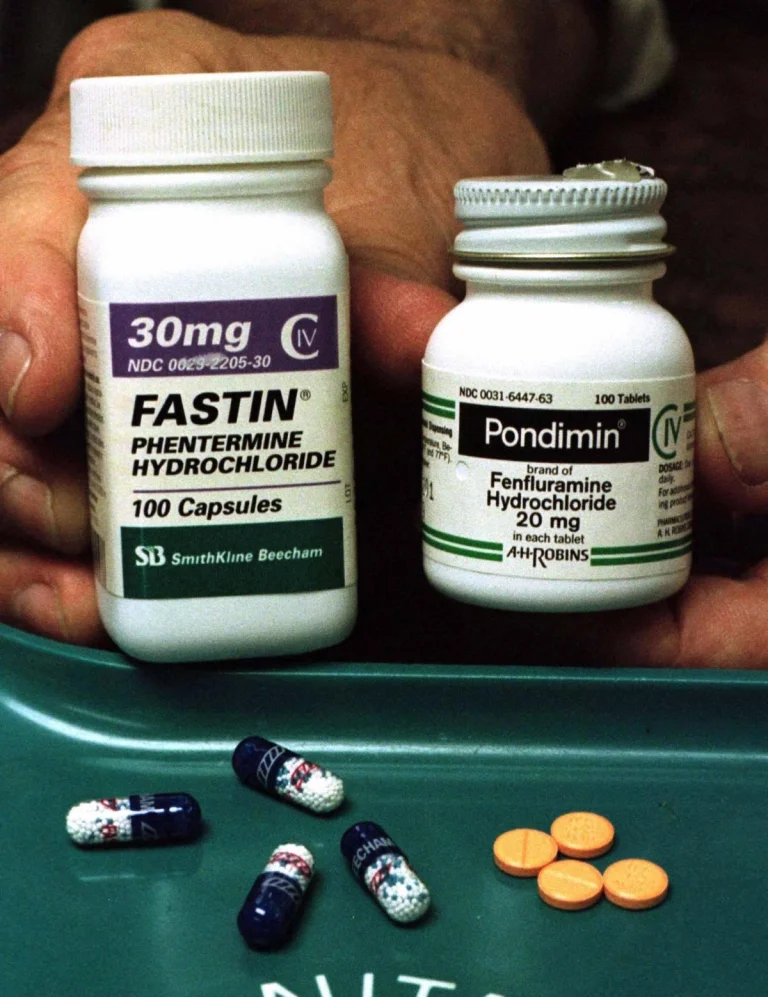
FEN-PHEN group consisting of Fastin (Fastin, Figure Left) and Fenflun (right) has swept the US weight loss pill market
Dr. Winterab, who combined the "Fen-Phen" group by a paper, then entered the FDA to work, and then an official at the FDA Pharmaceutical Review Center.
During his work in FDA, Wendrab used his right to provide a variety of policies for the diet clinic opened by his friend John Trevena. Winterab himself also authorized Treleta's clinic to use his image to promote weight loss products-of course, including "Fen-Phen".
With the help of Wentrab, Treletana got a large amount of promotion costs and prescription rebates from Fenflunin's manufacturers. The clinic business is very popular, and at the peak of the business peak period, more than 1,000 patients accept the weight loss plan provided by the clinic.
Thunderous
Just when people were ecstatic to find the "Fen-Phen" weight loss magic medicine, a bad news came without warning.
In 1995, the FDA received a listing application of the Redux preparation of Dexfenfluramine (Fenfluramine). Related pharmaceutical companies said that compared with the listing of Fenflumamin, Right Fen Fllaming has better curative effects and fewer side effects.
FDA review officials have found that in European clinical applications, Pendonin has reported high pressure on pulmonary arteries. As a fatal cardiovascular disease, the incidence of pulmonary hypertension itself is very low. Obesity is not a known risk factor for pulmonary hypertension. This made FDA review officials began to doubt that Lefen Flulaming was an independent factor that caused pulmonary high pressure.
However, under the "Fen-Phen" frenzy at the time, in the audit of the FDA expert group, Rightfen Fullaming passed the voting with a weak advantage of 6: 5 and was approved to be listed in 1996. Since then, Ryfen Fllaming quickly replaced Fenflunming in the market and became the "Fen" in "Fen-Phen".
In the year of the listing, Rightfan Fllaming won the sales performance of 1.2 million prescriptions. There are predictions that the market sales of Youfen Fllaming will reach $ 1 billion in the first five years of listing.
Youfen Flobumin did not survive the first five years after listing. On August 28, 1996, the authoritative medical journal "New England Medical Magazine" published an article, which reported that 24 cases of female patients who had heart valve disease appeared after taking "Fen-Phen". These patients do not have the risk factors of heart valve disease.
One day later, NEJM posted again that except for Finte Ming, a variety of appetite inhibitors, including Micharara, Fenfllaming, Finpreid (one of Fenflumamin) It can increase the risk of pulmonary hypertension by nearly 30 times, and this risk is independent of the basic condition of patients.
Subsequently, a large number of articles further confirmed this discovery. This forced the FDA to reintegrate the security of Fenflla Lamoming and Youfen Fllaming.
After weighing the advantages and disadvantages, the FDA made a decision on September 15, 1997 to revoke the listing permits of Fenflun and Youfen Fllaming.
Since there is no evidence, the use of fenming alone is related to pulmonary hypertension or heart valve disease, and after further review by the FDA, the drug can be retained in the market. But the popular "Fen-Phen" combination disbanded. REDUX withdrawn from the market because of the pulmonary hypertension and heart valve disease.
After the FDA decided to announce it, the major pharmaceutical companies that have been caught in the "Fen-Phen" frenzy were quickly rejected. A large number of patient groups filed a lawsuit against Fenflla Lamamin/Youfen Flulan. In 1999, the relevant pharmaceutical companies paid $ 4.83 billion in one -time compensation to resolve all lawsuits on Finfluorraming and Youfenflunamin.
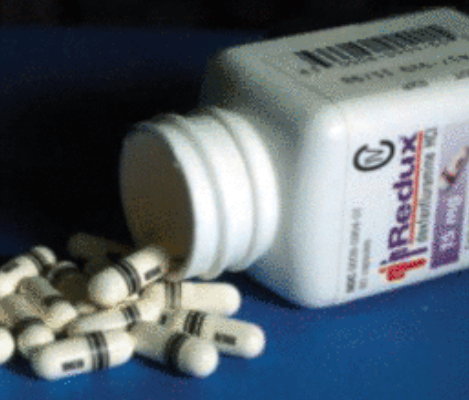
Ironically, Treleta, who once rely on "Fen-Phen", also brought the pharmaceutical company to court. Because he and his wife are loyal users of "Fen-Phen", they also suffer from severe heart valve diseases for medication.
To make matters worse, the company's radical marketing strategy has led to the disorderly expansion of Treer Leener's clinic and quickly closed down. Treleta was mixed in a state of bankruptcy.
Rise from the ashes
Fenfllaming causes pulmonary hypertension and heart valve disease. A large number of subsequent studies have shown that Fenflumin can activate the 5-hydroxylidin 2B receptor that exists on the heart valve, which stimulates the disorderly proliferation of heart valve cells, which eventually leads to the occurrence of heart valve lesions. It can also activate the pulmonary endogenic endothelial dermatotia. Type 5-hydroxyline 2 receptor on the cells induces pulmonary artery diastolic function damage and causes high pressure of pulmonary arteries. Due to the lack of activity to 5-hydroxylidine receptors, Finte Ming will not lead to these two pathological changes, so the serious side effects of the "Fen-Phen" combination are attributed to Fenflun Fluraramine.
In disaster and blessing, Fenfllaming is precisely because of the existence of neurotransmitted 5-hydroxylidin receptors, and it also ushered in the first-line vitality-treatment of epilepsy.
As one of the most difficult to treat epilepsy syndrome, the DRAVET syndrome is serious to existing antiepileptic drugs. Patients should control the condition and often need to take a variety of antiepileptic drugs to endure the huge side effects brought by drugs. For patients with DRAVET syndrome with cognitive disorder, it is undoubtedly worse.
The medical community's research on DRAVET syndrome has never stopped. In the phase III clinical trial, adding Finnonraming in basic therapy can reduce epilepsy by about 64%. This is undoubtedly good news for patients with DRAVET syndrome.
But how to balance the effects and cardiovascular risks of Fenflunradamin? This has become a major problem in regulatory agencies.
In the United States FDA requested that when Fenflun Ming was used to treat the DRAVET syndrome, the patient must receive the heart ultrasonic examination within 3-6 months after treatment, and within 3-6 months after treatment. It affects the heart valve. Fortunately, under this rigorous monitoring strategy, there is no case of heart valve disease caused by Fenflunin's treatment of Dravet syndrome.
It is not difficult to see from the example of Fenflunramumin that drug harm accidents are sometimes not an end to the development of drugs. On the contrary, under the circumstances of rigorous research on the pharmacological and toxic mechanism of drugs and fully preparing pre -clinical research, "toxic drugs" can also be transformed into "good medicines" for treating other diseases.
It is a good thing, but this kind of research consumes resources and energy. How many people dare to try it?
Reference materials:
[1] BALDWIN He, Nighland M, Kendall C, ET Al.40 Years of Topical Tretinoin Use in Review.j Drugs Dermatol.2013 Jun 1; 12 (6): 638-42.
[2] Donald G.Barceoux.Medical Toxicology of Drug Abuse: Synthesized Chemicals and Psychoactive Plants.john Wileysons.25-118-1060-1060-1060-1.
[3]Simon K,Sheckley H,Anderson CL,et al.A review of fenfluramine for the treatment of Dravet syndrome patients.Curr Res Pharmacol Drug Discov.2021 Dec 16;3:100078.doi:10.1016/j.crphar.2021.100078
[4] Bhattacharyya S, Schapira Ah, Mikhailidis dp, et al.drug-induced Fibrotic Valvular head disease.lance.2009 aug 15; 374 (9689): 10.1016/s016/s016730-30-670-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-30-670 X [5] AULT A.ANTI-OBESITY DRUGS Recalled from Global Market.the Lancet, 1997,350.9081: 867-867.
[6]RAY HM.The obese patient;a statistical study and analysis of symptoms,diagnosis and metabolic abnormalities;sex differences;treatment.Am J Dig Dis.1947;14(5):153-62.doi:10.1007/BF03001304
[7]Rasmussen N.America's first amphetamine epidemic 1929-1971:a quantitative and qualitative retrospective with implications for the present.Am J Public Health.2008;98(6):974-85.doi:10.2105/AJPH.2007.110593
[8] Parry HJ, Balter Mb, Mellinger GD, et al.NATIONAL PATTERNS of Psychotherapeutic Drug Use.arch Gen Psychiatry.1973 Jun; 28 (6): 18-74.
[9]Tragos CN.Fen-Phen Litigation Against American Home Products Corporation:The Widespread Use of Fenfluramine(Pondimin)and Dexfenfluramine(Redux)for Weight Loss,The Health Problems Associated with Those Drugs,the Resulting Litigation Against American Home Prod(2000 Third year paper). Harvard Law School Student Papers.
[10]U.S.Food and Drug Administration/U.S.Department of Health and Human Services Public Health Service,Reports of Valvular Heart Disease in Patients Receiving Concomitant Fenfluramine and Phentermine,FDA PUBLIC HEALTH ADVISORY,DEAR HEALTH PROFESSIONAL LETTER,July 8,1997.
[11] Weintraub M, Schuster B, ET Al.long-Term Weight Control Study.v (Weeks 190 to 210). Follow-up of Participants after CESSATION PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHLIN PHELIN PHELIN PHARIN. : 615-8.Doi: 10.1038/CLPT.1992.73
[12]Connolly HM,Crary JL,McGoon MD,et al.Valvular heart disease associated with fenfluramine-phentermine.N Engl J Med.1997;337(9):581-8.doi:10.1056/NEJM199708283370901[13]Manson JE , Faich Ga.PharMacotherapy for Obesity-Do the Benefits Outweigh The Risks? N ENGL J Med.1996; 335 (9): 659-60.Doi: 10.1056/Nejm199608293350910
[14] Kramer MS, Lane Da.aminorex, Dexfenfluramine, and Primary Pulmonary Hypertension.j Clin Epidemiol.1998 APR; 51 (4): 361-4.Doi: 10.1016/S0895-4356 (97) 00289-8-8
[15]Rothman RB,Baumann MH,Savage JE,et al.Evidence for possible involvement of 5-HT(2B)receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications.Circulation.2000;102(23):2836- 41.DOI: 10.1161/01.cir.102.23.2836
[16]BelohlávkováS,Simák J,KokesováA,et al.Fenfluramine-induced pulmonary vasoconstriction:role of serotonin receptors and potassium channels.J Appl Physiol(1985).2001;91(2):755-61.doi:10.1152/jappl .2001.91.2.755
[17]Lewis KH,Fischer H,Ard J,et al.Safety and Effectiveness of Longer-Term Phentermine Use:Clinical Outcomes from an Electronic Health Record Cohort.Obesity(Silver Spring).2019;27(4):591-602 .doi: 10.1002/OBY.22430
Source: Medical Community
Responsible editor: Wan Shunshun
School pair: Zang Hengjia
- END -
2021 China Micro Surgery Inheritance and Innovation Forum -successfully held the National Finals of the Case of Young Doctor

Sunshine News (Reporter Zheng Yalei) From June 16th to 17th, 2021 China Micro Surg...
Flat -bottomed shoes are not necessarily healthier, 3 kinds of shoes also hurt your feet
Everyone knows that high heels hurt their feet, so they usually wear comfortable a...