One article: CSCO Guide will be worthy of attention to the progress of rare target treatment progress
Author:Cancer Channel of the Medical Time:2022.06.19
*For medical professionals for reading reference
Directly hit the NSCLC guide update and research progress
In recent years, with the advancement of testing technology, more and more rare targets have been discovered, and new drugs in the opposite rare target have made rapid progress, giving rare non -small cell lung carcinoma (NSCLC) patients with rare drive gene positive. Gospel.
However, it is worth noting that NSCLC, which is rare gene mutation, still has unsatisfactory needs.
From April 23-24, at the China Clinical Oncology Society (CSCO) Guidelines, the update focus of the "CSCO Non-small Cell Cancer Diagnosis and Treatment Guide (2022)" (hereinafter referred to as the "2022 Guide") was announced. The molecular classification and treatment chapters of the 2022 version of the 2022 have been updated with rare target parts [1].
This article has been updated for NSCLC's rare target detection and treatment, and the treatment pattern inventory of related targets and the approval of new drugs are sorted and shared.
CSCO NSCLC diagnosis and treatment guide-
Rare target update part [1]
Molecular classification part: New "Met 14 Out -Wertzon Jumping Detection (Class 3)" is the II -level recommended III and IV -stage NSCLC.
2022 version guide
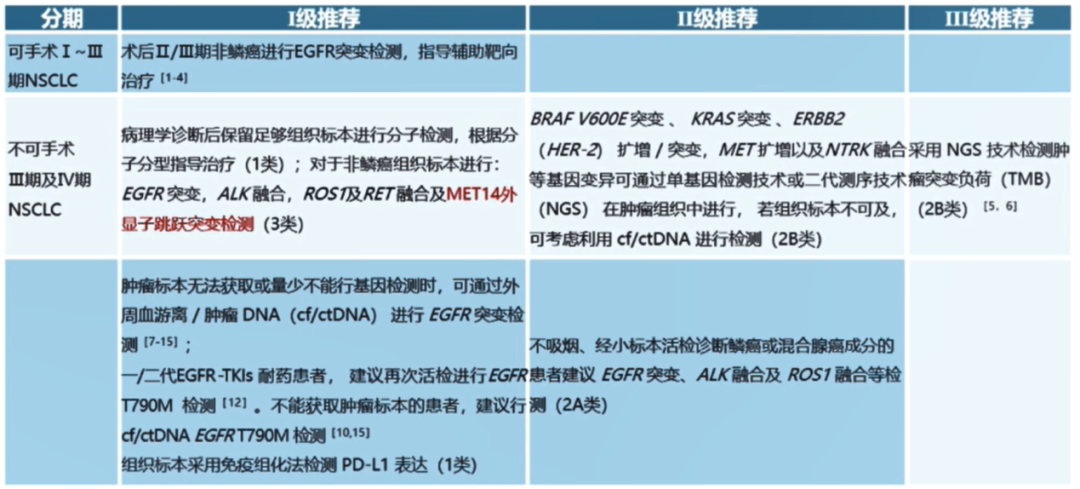
The revision is approved for listing based on MET No. 14 external jump mutation targeted drugs in China.
▍ Interpretation:
Met No. 14 outer appendal jump mutation is a rare drive gene mutation among patients with lung cancer. In the past, the treatment of lung cancer with jumping and mutations of Met 14 was mainly chemotherapy, but the curative effect was not ideal. The median total survival (OS) was only about half a year [1].
On June 22, 2021, the China National Drug Administration (NMPA) approved the MET inhibitors Savikinib for listing to treat the progress of platinum chemotherapy, or infective the standards of platinum chemotherapy, MET 14, No. 14 MET, No. 14 Patients for local advanced or metastatic NSCLC patients with jumping mutations. The approval was based on the study of the Meti -No. 14 outer sub -jump mutant NSCLC based on Savininib. The results show that the objective relief rate (ORR) evaluated by the Independence Review Committee (IRC) is 49.2%(95%CI 34.1%-62.3%), the disease control rate (DCR) was 93.4%(95%CI 84.1%-98.2%).
In addition, phase II VISION studies have shown that among the 152 MET14 out -of -revealed jumping mutations NSCLC patients who can evaluate the efficacy, the overall ORR treated with TEPOTINIB is 44.7%, of which the first treatment (n = 69) and the scriptures (N = 83) The patient's ORR was 44.9%and 44.6%[10]. Geometry Mono-1 Study on the Met14 Out-Ethnic Sub-Jumping Code data shows that the ORR of Capmatinib in patients (n = 28) is 67.9%, and the ORR in patients (n = 69) is 40.6%[11] Essence
Phase IV EGFR20 outer appetite insertion of mutation back line treatment: New "Mobocertinib" is recommended as III.
2022 version guide

The revision is based on the EXCLAIM research expansion queue and the patient (PPP) queue after platinum treatment.
▍ Interpretation:
EGFR20 outer slogan insertion mutation is a rare mutation in EGFR mutations. The incidence in the NSCLC of EGFR mutations is about 4%-12%[3], and the incidence in the overall NSCLC is about 2%[4]. Compared with the lack of mutations in the outer outer outerness of EGFR19 and mutation of the L858R dot point of the No. 21, the EGFR20 outer sub-insertion mutation is not sensitive to the first and second-generation EGFT-TKI.
The new Mobocertinib is based on a phase I/II clinical study of open labels, multi -centers, and phase 3 stages [4]. The study explores the treatment results and safety of Mobocertinib in the EGFR20 exogenous appetite insertion of the EGFR20 outerotic semirality in Platinum Chemotherapy [4]. The study and designing the dose, dose extension queue (PPP queue) and a single -arm extension queue (Exclaim queue).
According to the results updated at the 2021 American Clinical Oncology Society (ASCO) conference, as of November 1, 2020, in the PPP patient queue, the ORR confirmed by IRC was 28%(95%CI 20%-37%), researchers, and researchers The evaluation ORR is 35%(95%CI 25%-45%), the mid-bit relief duration (DOR) is 17.5 months (95%CI 7.4-20.3 months), and the median PFS is 7.3 months (95%95%(95% CI 5.5-9.2 months), the mid-position OS is 24 months (95Ci 14.6-28.8 months); in the Exclaim queue, the ORR confirmed by IRC is 25%(95%CI 17%-35%), and the researcher evaluates ORR is 32%(95%CI 23%-43%).
In terms of safety, the main adverse event is diarrhea. At present, Mobocertinib was approved by the US Food and Drug Administration (FDA) in September 2021 to treat adult patients with EGFR20 exogenous positive or transferred NSCLC adults for the treatment of platinum -containing chemotherapy. Rare targets that are worthy of attention to the progress of new drugs
In addition to the rare target drugs incorporated into the 2022 version guide, the CSCO Guide also shared the cutting -edge progress of some rare target drugs.
BRAF V600 mutation: Dalafini+Temin)
BRAF mutations occur in NSCLC about 1%-3%[5]. However, for this part of the patient, the effect of platinum dual -drug chemotherapy is poor, and the prognosis is usually not good.
Recently, Darafini Joint Qumei NMPA approved the treatment of BRAF V600 mutant metastasis patients. The approval of this combination therapy is based on the results of BRF113928.
BRF113928 is a multi -centered, multi -queue non -random open label research [6], exploring the efficacy of the Darafini ± aromitinib the treatment of BRAF V600 mutations NSCLC. The results showed that in the BRAF V600 mutant NSCLC's initial treatment and governance queue, the ORRs of Darafini+Temininib were 61%and 63%(IRC evaluation), respectively. Month (IRC evaluation).
HER2 mutation: piperkinib
A multi -centered, open label, and one -arm phase II clinical study [8] explored the efficacy and security of piperinib for HER2 mutations in late NSCLC. Studies were included in patients with advanced NSCLC patients who were incorporated into 60 HER2 mutations and at least first -line platinum chemotherapy. The main research final is the ORR evaluated by IRC.
The results of the study showed that ORR reached 30%(95%CI 18.8%-43.2%), and ORR was similar in patients with no brain metastases (25%vs 31.1%), and the median PFS reached 6.9 months (95%CI CI) 5.5-8.3 months), the mid-position OS reached 14.4 months (95%CI 12.3-21.3 months). The most common adverse events are diarrhea (20%, all level 3).
RET fusion: Selpercatinib
RET genes are more common in patients with lung adenocarcinoma without smoking, and the incidence in NSCLC is 1%-2%[5]. Libretto-321 Study [9] evaluated the effectiveness and safety of Selpercatinib (Loxo-292) in Chinese RET fusion-positive physical tumor patients. This study is included in RET fusion positive, and at least the first-line standard treatment/intolerance patients who have advanced diseases will be treated with SELPERCATINIB (LOXO-292) 160 mg BID treatment.
The main research groups include NSCLC patients (n = 47), and the central laboratory confirmed that RET fusion -positive NSCLC patients (n = 26), and the main research ending was the ORR evaluated by IRC. The results of the study showed that when the median follow-up time was 9.7 months, the ORR evaluated by IRC reached 69.2%(95%CI 48.2%-85.7%), and the 9-month DOR rate was 93.8%. Based on this study, SELPERCATINIB (LOXO-292) has been accepted by NMPA for listing and was included in the priority review by the China Pharmaceutical Review Center (CDE) of the China National Drug Administration.
references:
[1] Guide Working Committee of the China Clinical Oncology Society. China Clinical Oncology Society (CSCO) non -small cell lung cancer diagnosis and treatment guide 2022 [M]. Beijing: People's Health Publishing House.
[2]Gow,Chien-Hung et al.“A comprehensiveanalysis of clinical outcomes in lung cancer patients harboring a MET exon 14 skipping mutation compared to other driver mutations in an East Asian population.”Lung cancer(Amsterdam,Netherlands)vol.103 (2017): 82-89.doi: 10.1016/j.lungcan.2016.12.001
[3]Lu,Shun et al.“Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations:a multicentre,single-arm,open-label,phase 2 Study. "The Lancet.Respiratory Medicine Vol.9, 10 (2021): 1154-1164.doi: 10.1016/S2213-2600 (21) 00084-9 [4] fang, wenfeng et al. Response to osimtinib in non-syll lung carncer.
[5]Zhou,Caicun et al.“Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients With EGFR Exon 20 Insertion-Positive Metastatic Non-Small Cell Lung Cancer:A Phase 1/2 Open-label Nonrandomized Clinical Trial.”JAMA onCology Vol.7, 12 (2021): E214761.doi: 10.1001/jamaoncol.2021.4761
[6]. Yang Guangjian, Wang Yan. Non-PFS lung cancer rare gene mutations in the treatment research progress [J]. Cancer progress, 2019,17 (12): 1371-1376,1418.
[7]Odogwu,Lauretta et al.“FDA Approval Summary:Dabrafenib and Trametinib for the Treatment of Metastatic Non-Small Cell Lung Cancers Harboring BRAF V600E Mutations.”The oncologist vol.23,6(2018):740-745.doi : 10.1634/theonCologist.2017-0642
[8] li B, Smit EF, Goto Y, ET Al.primary Data from Destiny-LUNG01: A Phase 2 TRIAL of Trastuzumab DeruxTecan (T-DXD) In Patients (PTS) Mutastic Non-SON-SONTALAL NON -S Cell LUNG CANCER (NSCLC) .presented at: 2021 ESMO Congress; SEPTEMBER 16-21,2021; virtual.abstract LBA45.
[9]Zhou,Caicun et al.“Pyrotinib in HER2-Mutant Advanced Lung Adenocarcinoma After Platinum-Based Chemotherapy:A Multicenter,Open-Label,Single-Arm,Phase II Study.”Journal of clinical oncology:official journal of the American Society of clinical onCology Vol.38,24 (2020): 2753-2761.doi: 10.1200/JCO.20.00297
.Clin Cancer Res (2022) 28 (6): 1117–1126.
[12] Wolf J, Garon Eb, Harry JM, ET Al.capMatinib in Met Exon 14-Mutated, Advanced NSCLC: Updated Results from the Geometry Mono-1 Study.j Clin Oncol 2021; 39 (SUPPL_15): 9020-9: 9020-9: 9020-9: 9020-9: 9020-9: 9020-9: 9020-9: 9020-9: 9020-9:
This material is provided by Astrikon, for reference for medical professionals
Cn-96453
This article is only used to provide scientific information to medical professionals, which does not represent the platform position
Click "Read the original text" to view


- END -
Orange Light Class — Professional help children grow diverse growth

Orange Light Class is a service platform that integrates public welfare, resources...
Good medical sound daily science 丨 international anti -drug day: healthy life green and non -toxic

June 26 this year is the 35th International Anti -Drug Day. This year's theme is H...