These drugs have suspended sales and recalls!
Author:Jiangsu News Time:2022.06.21

The latest announcement issued by the State Drug Administration
Involved in "Children's Cold Granules"
"Chuanbei cough syrup" and so on
Nine companies produced
10 batches of medicines do not meet the requirements

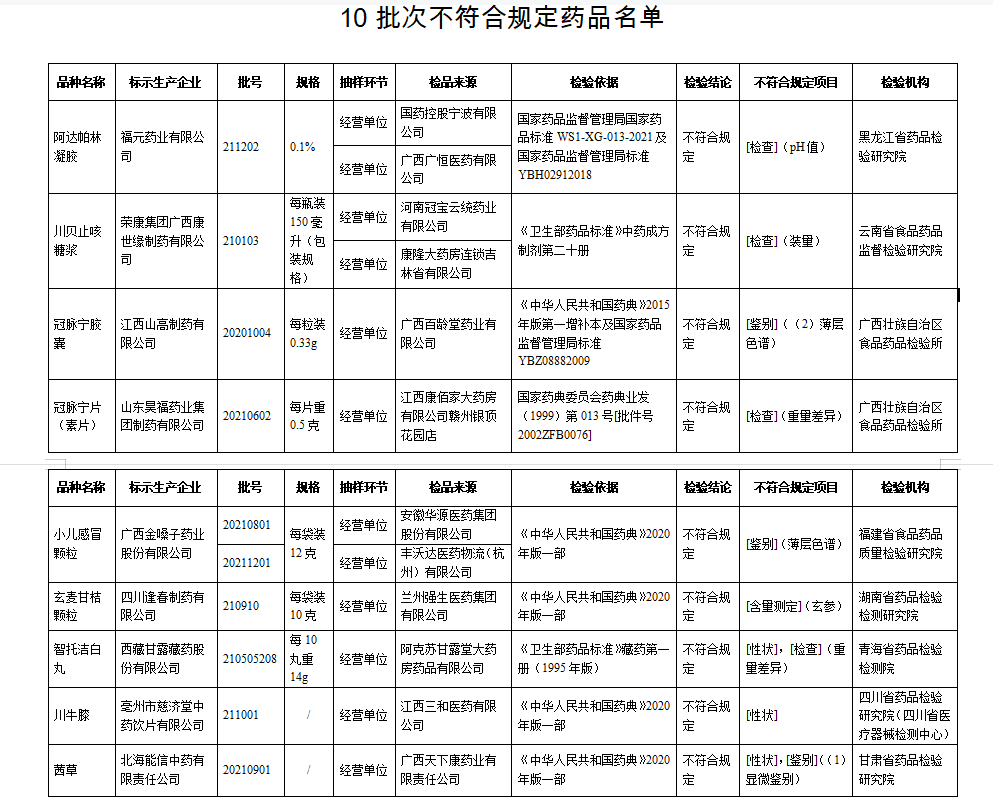
1. After inspection by Heilongjiang Pharmaceutical Inspection and Research Institute, a batch of Adapolin gel produced by Fuyuan Pharmaceutical Co., Ltd. does not meet the regulations and does not meet the requirements of the specified project as a pH value.
2. After inspection by the Institute of Food and Drug Supervision and Inspection of Yunnan Province, a batch of Chuanbei cough syrup produced by Rongkang Group Guangxi Kangshiyuan Pharmaceutical Co., Ltd. did not meet the requirements and did not meet the requirements of the specified project as the amount of installation.
3. After inspection by the Food and Drug Inspection Institute of Guangxi Zhuang Autonomous Region, a batch of coronary Ning Ning capsules produced by Jiangxi Shan Gao Pharmaceutical Co., Ltd. do not meet the regulations and do not meet the requirements of the regulations as a identification; The 1 batches of coronary Ning tablets (vegetarian tablets) do not meet the requirements, and it does not meet the requirements of the specified project as a weight difference.
4. After inspection by the Food and Drug Quality Inspection and Research Institute of Food and Drug Quality, the two batches of children's cold particles produced by Guangxi Golden Vol.
5. After inspection by the Hunan Pharmaceutical Inspection and Inspection and Inspection and Institute, a batch of Xuanmai Ganpen Persians produced by Sichuan Fengchun Pharmaceutical Co., Ltd. did not meet the requirements and did not meet the requirements of the specified project as the content measurement.
6. After inspection by the Qinghai Pharmaceutical Inspection and Inspection Institute, a batch of Zhito Jie -Bai Pills produced by Tibetan Ganlu Tibetan Pharmaceutical Co., Ltd. did not meet the requirements, and did not meet the requirements of the specified projects as traits and weight.
7. Inspection by the Sichuan Pharmaceutical Inspection and Research Institute (Sichuan Medical Device Inspection Center), a batch of Sichuan Achyranthes of Sichuan Achyranthes does not meet the requirements and does not meet the specified projects as traits.
8. After inspection by the Gansu Pharmaceutical Inspection and Research Institute, a batch of Qiancao produced by Beihai Nengxin Traditional Chinese Medicine Co., Ltd. does not meet the requirements, and does not meet the requirements of the specified projects as traits and identification.
For the above -mentioned do not meet the prescribed drugs, the drug supervision and management department has required relevant enterprises and units to take risk control measures such as suspension of sales and use, recall, and conducting investigations and rectification on reasons that do not meet the prescribed reasons.
The State Drug Administration requires relevant provincial drug supervision and management departments to organize investigations on suspected illegal acts existing above -mentioned enterprises and units in accordance with the Drug Administration Law of the People's Republic of China, and publicize the results of the results in accordance with regulations.

Source/ National Drug Administration
Edit/Yuanyuan
© Jiangsu News
focus on
- END -
Is the scorching sun burning?Suggestion: Drink 5 "Runyan Tea", refreshing and moisturizing, fairness in summer

Summer is here, the clear sky is clear, the scorching sun is burning, and the sun ...
The surgery has not yet begun, and the patient's breathing is severe.

At eleven o'clock at night, the emergency surgery notice from general surgery was ...