Where should the patients with a rare ALK double fusion mutation NSCLC go from?And look at this case report
Author:Cancer Channel of the Medical Time:2022.06.23
*For medical professionals for reading reference
Interpret a diagnosis and treatment of NSCLC patients with double fusion of EML4-ALK BIRC6-ALK.
Lung cancer is one of the most common malignant tumors. Non-small cell lung cancer (NSCLC) accounts for 80%-85%of lung cancer. With the continuous deepening of the research of the NSCLC pathogenic mechanism and the discovery of driving genes, targeted therapy significantly improves the late-stage drive gene positive late stage NSCLC's prognosis [1]. ALK fusion is an important driving gene for NSCLC, which can benefit from the ALK inhibitor treatment. ALK fusion has a variety of partner genes. In addition to the common EML4-ALK, more and more rare fusion partners have been reported. In clinical treatment, the attention of the treatment of ALK rare fusion NSCLC patients has gradually increased.
It has important clinical significance to continuously explore the new ALK fusion form and study its correlation with drug sensitivity. At present, more than 90 more rare ALK fusion has been found in NSCLC, including KIF5B-ALK, HIP1-ALK, TPM-3ALK, etc. [2,3]. In 2020, the LUNG Cancer magazine reported a patient with EML4-Alk Birc6-ALK dual fusion and was sensitive to the second-generation Alk-TKI [4].
EML4-ALK BIRC6-ALK dual fusion patients are sensitive to Alaitinib
The patient is a 60 -year -old man. In December 2019, a regular medical examination, CT showed a mass with a size of 2.3 CM x 3.1 cm in the front of the right upper lung, merged with multiple lung diversions, and hyperthyroidism. After lung biopsy, the pathological diagnosis is right lung adenocarcinoma (T4N3M1A, IVA stage).
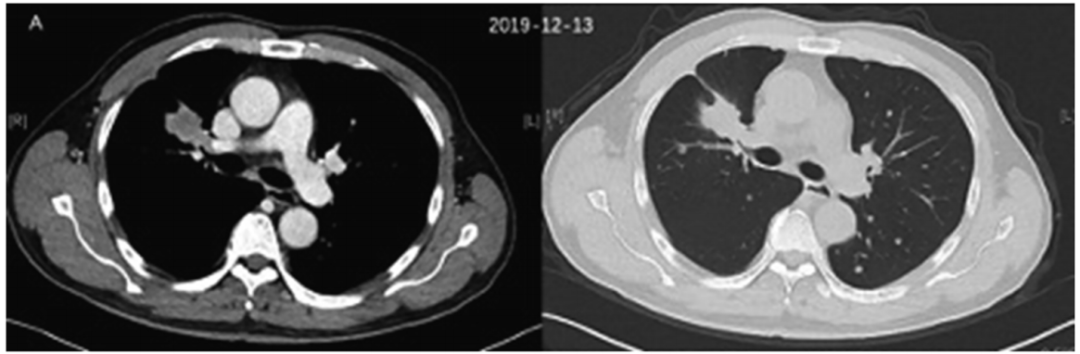
Figure 1. CT inspection results in December 2019
NGS shows that there is ALK dual fusion mutations: EML4-ALK (E20: A20, 2.14%in the organization) and Birc6-ALK (A19: B33, 3.42%in the organization). Since January 2020, the patient began to take Alaitinib with a dose of 600mg, twice a day. After 2 months of treatment of Alaitinib, the CT review in March 2020 found that the lesion was reduced from 2.3 CM × 3.1 CM to 1.7 cm × 2.3 cm.
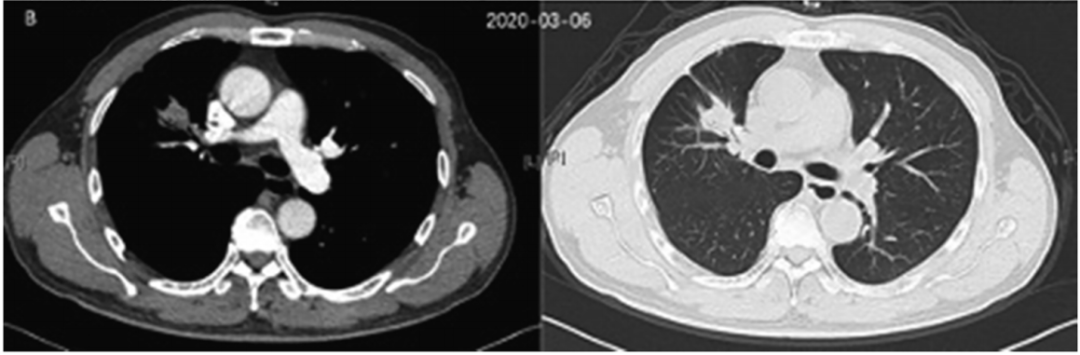
Figure 2. March 2020 CT inspection results
Detecting plasma circulatory tumor DNA (CTNDA) through liquid biopsy, and found that the ALK fusion gene disappears. As of April 2020, patients were still taking Alaitinib, and no obvious drug -related adverse reactions were found.
Other ALK fusion is also sensitive to Alaitinib
Research data showed that the efficacy of the first-generation Alk-TKI drug Cizidinib, and the second-generation Alk-TKI drug Bogntinib and Sereidib may be affected by the EML4 variant type, treating the objective of the V3 variant patients Data such as relief rates (ORR) and no progressive survival (PFS) are generally lower than those of V1 variants. For example, the median PFS of Cizidinib the treatment of EML4-ALK V1 variants and V3 variants is 11.0 months and 4.2 Month (P <0.05) [5-8]. Alaitinib showed a more consistent curative effect in different ALK fusion mutations. The results of the BFAST [9] research show that for the ALK fusion patients for EML4-V1 or V3 variants A good response rate, the patient's ORR is about 90%. In addition, in the research results, Alantini also showed good therapeutic effects among patients with ALK fusion partner gene non-EML4 (NON-EML4-ALK), and ORR reached 79%.
And in other rare mutations, such as HIP1-ALK fusion, StRN-ALK fusion, LINC00478/LINC01549, and ALK outer 20-generic 20 generic region fusion, A Laidinib also achieved a good effect [10- 12]. A case published in 2021 LUNG CANCER shows that [10], HIP1-ALK fusion may lead to the treatment of primary resistance for cizotininib, and A Laitinini treats HIP1-ALK for the primary resistance of cizininininin The patient continued to relieve, and the mid -position PFS exceeded 9 months. A case published in 2020 JTO Clin REP shows that [11], StRN-ALK (fusion breakpoint S3, A20) patients received Alaitinib for 6 consecutive months to continue answering. occur. A case published in the 2020 JTO Clin REP shows that [12], for patients with a genetic regional fusion of LINC00478/LINC01549 and ALK outer 20, Alaitinib can also bring clinical benefits. At the time of the data submission, the patient's median PFS after receiving Alaitinib for more than 6 months.
summary
About 5%of lung adenocarcinoma exists in ALK gene fusion, but the incidence of ALK dual fusion is low and related data is small. So far, only a few cases have been reported. In clinical treatment, the rare fusion of ALK is continuously increasing. This is the first report of EML4-Alk Birc6-ALK dual fusion cases. Patients have achieved good results through the second-generation TKI Alaitinib therapy. In addition, for other rare fusion (such as StRN-ALK fusion and LINC00478/LINC01549), A Laidinib has achieved good results. Expert Introduction
Professor Yu Jiangyong

Doctoral post -doctoral tutoring tutor for doctoral tutors
Deputy Chief Physician of the Department of Cancer Department of Beijing Hospital
He is currently served as executive member and secretary of the Professional Committee of the Beijing Oncology Society, a member of the China Clinical Oncology Society (CSCO) Tumor Nutrition Treatment Expert Committee, a member of the Cancer Chemotherapy Professional Committee of the China Human health and Technology Promotion Association, a member of the Professional Committee of the Pulmonary Cancer Immunotherapy of the Beijing Cancer Prevention Society. Member of the Beijing Medical Reward Foundation of the Pulmonary Cancer Medical Youth Expert Committee, a member of the Beijing Breast Disease Prevention and Treatment Society of Tumor Immunotherapy, the first member of the Beijing Integrated Medicine Society, and the first member of the Beijing Anti -Cancer Association Popular Science Professional Committee.
Professional direction: precise diagnosis and treatment of lung cancer and individual chemical treatment. Since 2019, he has presided over a special project on the National Natural Science Foundation of China and one project on the National Natural Science Foundation of China. , SCRT, Cancer Lett, and Cancer Med published SCI papers.
references:
[1]. Zhang Tao. Molecular targeted drugs are used in the research progress of the maintenance treatment of non-small cell lung cancer [J]. Chinese lung cancer magazine, 2010,13 (11): 1070-1073.
[2] .takita, J. The Role of Anaplastic Lymphoma Kinase in Pediatric Cancers. Cancer SCI 108, 1913-1920. (2017).
[3]. Katayama, R. Et Al. Therapeutic targeting of anaplastic lymphoma kinase in Lung Cancer: a Paradigm for Precision Cancer Medicine. CLIN CANCER Res 21, 2227-2235. (2015).
[4] .zhong jm, zhang gf, lin l, li dy, liu zh. A novel eml4-alk 6-alk diety variant in lung adenocarcinoma confers sensitivity to alectinib. Lung Cancer.
[5].Christopoulos P, Endris V, Bozorgmehr F, et al. EML4‐ALK fusion variant V3 is a high‐risk feature conferring accelerated metastatic spread, early treatment failure and worse overall survival in ALK+ non‐small cell lung cancer[J ]. International Journal of Cancer, 2018, 142 (12): 2589-2598.
[6].Yoshida T, Oya Y, Tanaka K, et al. Differential crizotinib response duration among ALK fusion variants in ALK-positive non–small-cell lung cancer[J]. Journal of Clinical Oncology, 2016, 34(28) : 3383-3389.
[7].Camidge D R, Niu H, Kim H R, et al. Correlation of baseline molecular and clinical variables with ALK inhibitor efficacy in ALTA-1L[J]. Journal of Clinical Oncology, 2020, 38(15_suppl): 9517.[ 8] .cha y j, kim H r, shim H s. Clinical outcoms in alk-rearranged lung adenocarcinomas account to alk fusion variants [J]. Journal of translational media, 2016, 14 (1): 1-10.
[9].Dziadziuszko R, Mok T, Peters S, et al. Blood First Assay Screening Trial (BFAST) in Treatment-Naive Advanced or Metastatic NSCLC: Initial Results of the Phase 2 ALK-Positive Cohort. J Thorac Oncol. 2021; 16 (12): 2040-2050.
[10] .li M, Tang Q, Chen S, et al. A Novel Hip1-ALK FUSION VARIANT in LUNG ADENOCARCINOMA Showing Resistance to Crizotinib [J]. LUNG CANCER, 2021, 151: 98-100.
[11]. Nagasaka M, SARVADEVABATLA N, iWata S, et al. Strn-alk, a Novel in-Frame Fusion with Response to Alectinib.
[12] .peng w, li s, li l, xiao m, zhong j. a novel linc00478/linc01549 intergenic region-alk fusion responded well to alectinib in a patient with adenocarcinoma. ): 100112.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
Luo Ding: "Children's House" escort minors grow up healthy growth

A few days ago, the propaganda activities of the Protection Law of the People's Re...
Who can cross the cycle?"Legend" of China CAR-T therapy

Legendary creature was founded in Nanjing in 2014 and was incubated by the world's...