What is the effect of the 1.2 million needle?
Author:Look at the think tank Time:2022.06.25
On June 22, 2022, the first CAR-T cell therapy product Akilun Sai (product name: Yi Kaida) was approved for listing in China for just one year. What is the treatment effect of tumor patients treated this year? What is the first patient in China? Originally at its own expense of 1.2 million yuan per drug, did the medical burden of patients be alleviated now?
According to previous reports, the Aquelon injection is mainly used to treat patients with recurrence after second -line or above systematic treatment, or cure patients with unpleasant B -cell lymphoma. Large B -cell lymphoma is one of the most common types of non -Hodgkinoma. Non -Hodgkin lymphoma is one of the more common adult hematoma -lymphoma. Reunion and drug resistance are a major problem facing lymphoma patients.
Since June 2021, Ruijin Hospital affiliated to the School of Medicine of Shanghai Jiaotong University passed the treatment of "Yicaida" CAR-T cell therapy for a "diffuse large B-cell lymphoma" patient. The patient was inferred, and the patient was evaluated by PET-CT imaging, and the symptoms were completely alleviated.
The above case is also the first patient in China to be treated with CAR-T. What is her treatment now?
Wen | Chen Sisi
This article is reproduced from the WeChat public account "Surging News" (ID: ThePapernews). The original first was released on June 23, 2022. The original title was "What is the effect of anti -cancer drugs for 1200,000 needles? "The first CAR-T treatment patient in China" does not represent the viewing think tank view.
1
The first patient in China:
Three follow -up after losing treatment shows that the tumor symptoms continue to relieve
"From August (last year) to the present, the treatment has been nearly 11 months. From the three follow -up after discharge, the current condition has been relieved, and there are no symptoms such as fever. Everything is good." CAR-T patients Aunt Chen said that a total of 3 follow-up was followed by the 28th day after the return, and the following 3 months and 6 months, the follow-up examination showed that the symptoms were completely relieved.
Aunt Chen was a foreign patient. Due to the epidemic of Shanghai, she failed to go to Ruijin Hospital for a follow -up of the hospital for the launch of the hospital. It is expected that she will go to Shanghai in August 2022 to complete the one -year follow -up.
On June 23, Professor Xu Pengpeng, deputy chief physician of the Hematology Department of Ruijin Hospital, revealed to reporters that after the patient lost CAR-T cells, the tumor control was generally good. During the treatment, only slight body temperature increased, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this, but this was this increased, but this was this, but this was this increased, but this was this increased, but this was this, but this was this, but this was this, but this was this. It's just an excessive temperature drop after control. On the 28th day after infusion, imaging examinations show that the activity of tumors has been completely controlled. 3 months and 6 months after treatment, the examination showed that the tumor was completely relieved.
"If the patient reaches the state of continuous relief for more than 3 months, it means 90%of the patient can be completely cured." Xu Pengpeng said that it still cannot be said to be 100%cure. The patient will continue to be followed in the future. treatment effect.
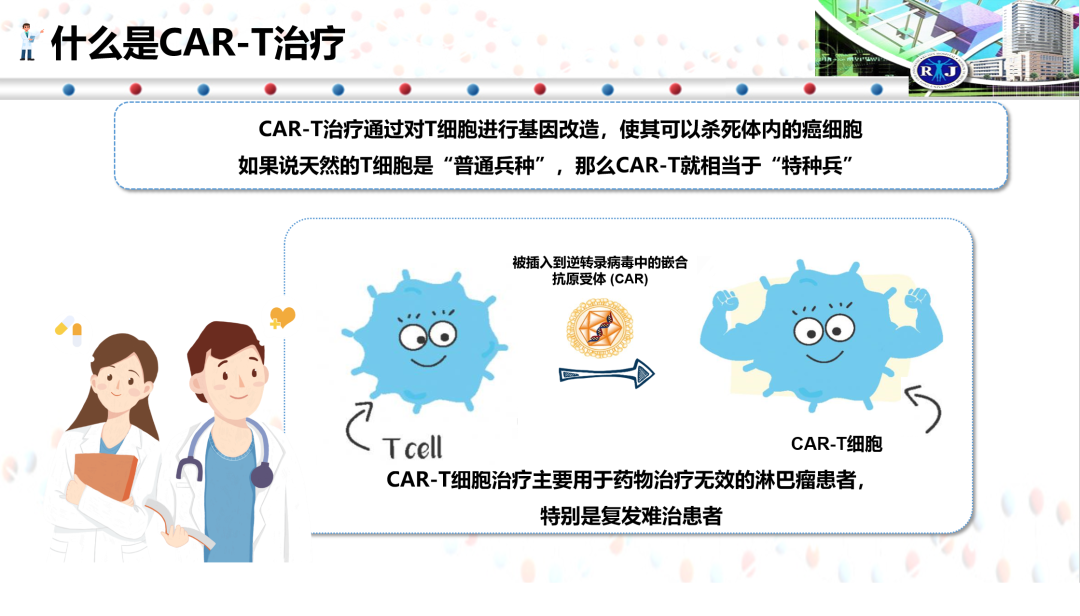
CAR-T the treatment of the pictures of this article is provided by the respondents
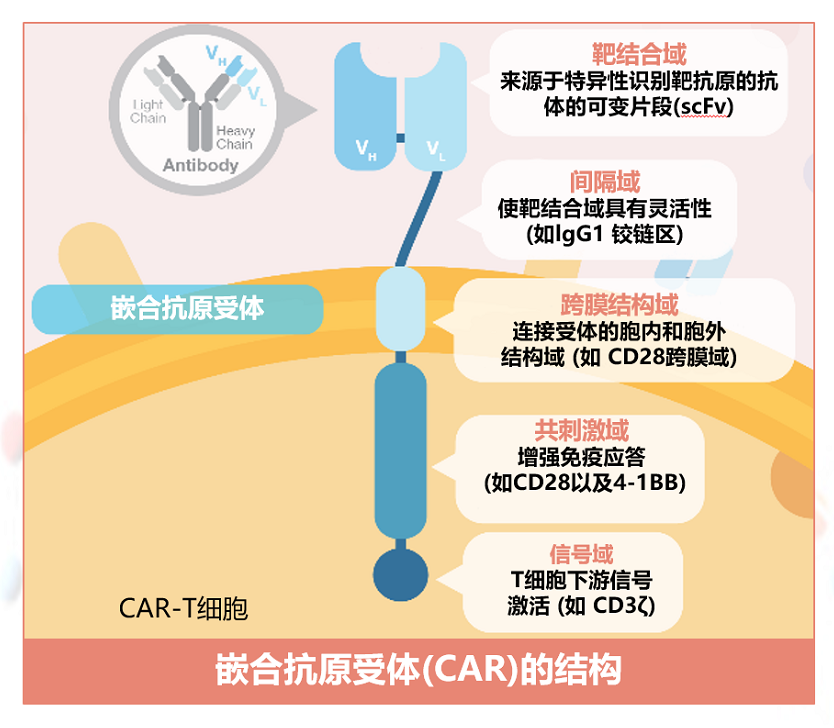
The structure of the chimeric antigen receptor (CAR)

CAR-T therapy
Xu Pengpeng said that as of June 2022, more than 30 patients in Ruijin Hospital had accepted the collection of Yi Kaida cells, and some patients had completed treatment. At present, 19 patients have completed the first efficacy assessment. After the management of the patient's full process, the total treatment efficiency reached 94.7%, which is much higher than the results of foreign research and the real world of American real world.
Professor Wang Li, chief physician of the Department of Hematology of Ruijin Hospital, also mentioned another case of a large B-cell lymphoma patient treated with "Yicaida" CAR-T cells in the hospital. Many complications such as drying syndrome, after 3 months and 6 months of conditional assessment, are still the state of continuous relief of tumor symptoms.
A data from Fosun Kate shows that the first anniversary of Yikaida's listing has benefited more than 200 patients with lymphoma. "From the perspective of foreign and domestic studies, for patients with large B -cell lymphoma patients with chemotherapy treatment resistance, if they do not continue to obtain good drug curative effects, continue to use large -dose chemotherapy combined with autologous transplantation methods, they The median survival is only about 6 months, and currently CAR-T treatment is the best treatment plan for these patients. "Wang Li said that foreign Car-T research shows that if patients can last 3 months of symptoms, the symptoms can be relieved, and the symptoms can be relieved. His subsequent tumor removal rate is above 90%, so 3 months are very important.
What kind of tumor patients will recur within 3 months? Wang Li explained that first is to be related to the degree of malignant tumor. The more gene mutations, the higher the malignant tumor, and the possibility of recurrence; the second is the load of the tumor. To reduce the load of the patient's tumor, the smaller the complications that occur during CAR-T treatment; the third is patients with symptomatic relief, and they will also recommend that they continue to take the "Zabitinib" drug to effectively enhance the amplification of T cells At the same time, it effectively reduces the release of immunosuppressive cells in patients and the release of cytokines in immunosuppressive cells, making CAR-T therapy more effective and reduced recurrence as much as possible.
2
treatment fee:
Including more commercial insurance
In October 2021, due to the high treatment cost of 1.2 million yuan per need, CAR-T cell therapy products were widely concerned after the listing of products.
At present, does the burden on the patient's medication be alleviated?
In this regard, Huang Hai, CEO of Fosun Kate, revealed to reporters: "From the initial stage of self -funded to corporate assistance, as well as commercial insurance, including urban custom insurance, etc., in order to reduce drug prices and increase the availability of patients' medication, the enterprise also made it. Multi -level guarantees. As of May 25 this year, 'Yikaida' has been included in more than 30 benefits of people's protection, and more than 50 commercial insurance institutions have included Yi Kaida in the reimbursement list, covering Ping An Life, covering Ping An Life, Ping An and Health, Zhong'an Insurance, Fosun United Health, etc. "
At the same time, some cities across the country have also incorporated CAR-T products into urban custom insurance. On June 9, 2022, Surging News reported that the 2022 version of "Huhui Insurance" broke through the new CAR-T high-headed medical security to enhance the availability of innovative drugs, allowing the world's high-end medical technology to benefit more patients, and 0 Demolition, the maximum payment amount is 500,000 yuan.
Huang Hai further revealed that, in addition to Shanghai's Huhui Bao, at present, Xiangshan Bao, Yunfu Hui Yunbao, and Shenzhen Pengcheng Insurance in Zhongshan, Guangdong also include Yicaida into it, and more patients can benefit.
How to protect the patient's CAR-T treatment during the epidemic?
Huang Hai responded that CAR-T treatment is not a traditional treatment method, which requires the process of cell collection and production and preparation, involving more than 600 processes, and the cell preparation production plant is located in Zhangjiang, Pudong. We changed the original high -speed rail transportation to highway transportation. Our patients from all over the country and needed to transport the prepared cells to the city where the patients are located. During the epidemic, it provided the guarantee of the drug supply chain for patients in Guangzhou, Chengdu, Beijing, etc. It was also supported by the government and all sectors of society. One of the patients in Beijing was dangerous. The Beijing Pharmaceutical Supervision Bureau helped the drug transportation of the patient alone. Special channels have guaranteed CAR-T treatment of dozens of patients. "
Huang Hai also said that in the future, he also hopes to establish more CAR-T cell therapy centers like Rijin Hospital in the country to benefit more patients. As of the end of May 2022, more than 80 therapeutic centers in Yi Kaida have been filed. It is expected that it will continue to expand in 2022. It is expected to exceed 100 to meet the recurrence and refractory large B -cell lymphoma patients in different provinces and cities. Treatment of healing.

- END -
A neck pattern is 10 years old, and lapherbopic thyroid surgery will solve your worries for you

Since ancient times, there have been traditions of praising women's necks. They of...
Which medicines are expected to enter medical insurance?How to renew the contract?
The adjustment of the medical insurance directory is related to each insured. The State Medical Insurance Bureau recently issued the National Basic Medical Insurance, Work Injury Insurance and Matern