SMA oral pharmaceutical Liste Polan's price reduction!Early diagnosis and early treatment highl
Author:Pharmaceutical economy Time:2022.06.10
In recent years, the state has vigorously promoted the cause of rare disease prevention and treatment, which has brought more innovative drugs to patients with rare diseases including spinal muscle atrophy (SMA) in China, and explores 1+N's rare diseases. The hierarchical medical security system has continuously improved rare medical drug protection.
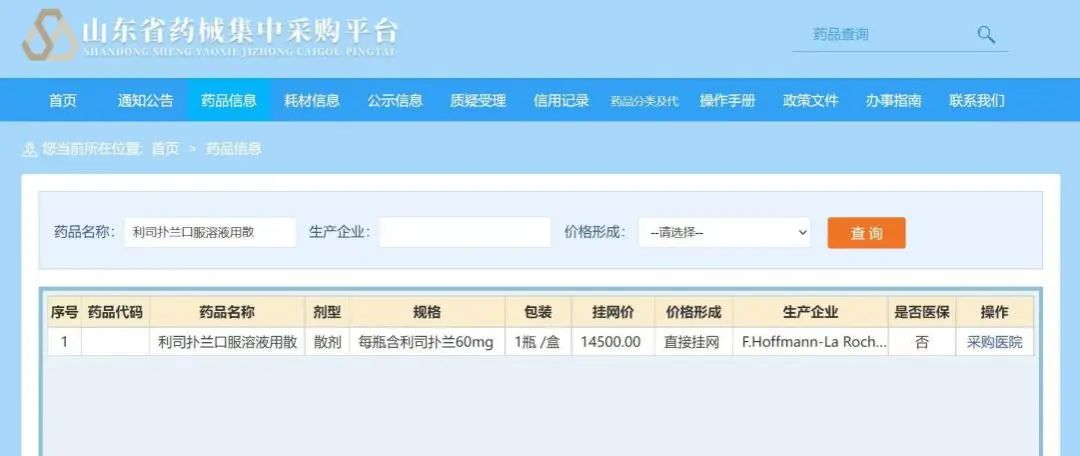
In order to further reduce the treatment of patients and families with SMA patients and families, and enhance the visibility of SMA treatment, on June 9, 2022, the price of Lisi Poland's oral solution (product name: Ai Manxin) was officially official Setting down, the price of the Shandong province's recruitment platform was the first to show that the price dropped to 14,500 yuan per bottle. Because the liberalized solution of the Lisu Panlan mouth is administered at age and weight, after the price adjustment, the maximum dose patient (2 years old and over 20 kg), the burden on the treatment of the year of Lisuland will be less than 450,000 yuan. , Low -weight patients have a lower economic burden, and the annual treatment costs are more than 2/3 of the maximum dose.
SMA is a rare, life -threatening disease. Most of the children died within the age of 2, and the median survival period was about 10 months. The main genetic disease in the field of defects leads to the death of infant death. The incidence of SMA is 1/100,000 of surviving newborns. About 1,000 new SMA patients are added each year, and about 80%of them will get onset within 18 months after birth. With the development of SMA's condition, the skeletal system, respiratory system, digestive system and other systems are abnormal, endangering patient life, and also bring a heavy psychological and economic burden to the patient's family. Clinical treatment needs to enrich innovative drugs and solutions to further improve Patients have long benefited.
Reduce the cost and risk of doctors and patients
Effectively improve the clinical benefit of patients
The first batch of rare disease catalogs jointly formulated by the five departments such as the National Health and Health Commission Among them, SMA has been officially included in it, improving the availability of drugs and improving the burden of drugs. It has received more and more attention and attention at the national level.
With the continuous listing of innovative drugs, SMA's clinical treatment has been significantly improved. At present, the traditional treatment plan requires patients to inject in -shell injection in the form of lumbar spine puncture in a specific hospital. From the perspective of the hospital, the internal injection of the sheath involves repeated surgical operations and usually requires sedative and imaging guidance. This requires specific ones. Professional medical center for medical professional knowledge and equipment.
Patients and their families regularly go to such medical centers. In addition, corresponding postoperative management is undoubtedly a major connection burden for the family of SMA patients, which greatly increases the cost of direct medical resources, and transportation, accommodation and lodging The indirect costs such as mistakes and misunderstandings may increase the risk of adverse reactions such as headache, back pain, vomiting, and severe infection.
Category 1 Innovation Pharmaceutical Lisi Panlan Pleurthropic solution is based on the treatment of spinal muscle atrophy (SMA) for the rapid approval of my country's rare disease regulatory policy.Clinical needs and drugs can be obtained, adding new treatment options for patients.
Experts said that from the perspective of the patient, oral administration means that patients can treat home treatment, which has greatly improved the convenience of treatment for patients with inconvenient action or paralyzed for a long time; About 80%of patients were onset within 18 months after birth, and patients were younger at the time of onset. Compared with invasive surgical treatment, oral administration greatly increased the compliance of children's medication, which was conducive to reducing the risk of treatment.
It is understood that in clinical practice, the administration method and pharmaceutical dosage form have a certain impact on the treatment compliance of patients, especially children; Lumbar puncture often needs to be performed under the guidance of ultrasound or magnetic resonance, and creative operations are also very painful for patients.
The efficacy and safety of drug stability also ensure the sustainability of SMA long -term standardized treatment and follow -up, thereby effectively improving the clinical benefits of patients. Oral administration methods are more advantageous in terms of convenience and compliance, reflected at the economy level, bringing greatly reduced treatment costs. In addition to bringing revolutionary changes to clinical treatment, disruptive rare disease treatment techniques also need to pay more attention to the availability and payment of rare disease drugs.
The cost -effective cost can be controlled
urgently need to further improve the burden on drugs
my country's medical insurance payment system has been continuously reformed. The level of clinical treatment has brought hope, and the diversified payment system around rare diseases and orphan drugs is being formed.
At present, SMA patients and their families still have a large economic burden. SMA patients have a serious complications and have a low quality of life in themselves and their families. On average, one patient requires at least 2 careers, and existing drug treatment is limited, and there are still large and unsatisfied treatment needs clinically. It is worth looking forward to that the use of Lispan's oral solution to enter medical insurance payment will bring greater clinical benefits to Chinese SMA patients.
In terms of efficacy, Liste Poland's oral solution was scattered as the first small molecular disease correction treatment drug that targeted MRNA. Compared with the therapy acting alone on the central nervous system, more benefits can be achieved.
A variety of clinical research results also showed that the use of Liste Poland's oral solution can effectively extend the survival period of SMA patients, improve exercise functions, and bring more whole body benefits to patients, and good safety; now Some SMA treatment schemes still have shortcomings: in the sheath injection is limited in patients with spine bending, poor respiratory function, and unstable diseases. For such patients, the use of Liste Puoran's oral solution provides a new and better treatment option to further improve the level of rare diseases.
inIn terms of cost control, the use of Lispan's oral solution is also more economic. Due to the use of steps doses, Liste Polan's oral solution is very small for patients with scattered age and small weight patients. The annual treatment cost is more than 2/3 of the dosage. After that, the dose is constant, and it will no longer continue to increase with age weight. In short, the younger the patient's medication, the lower the burden of treatment, and the better the early treatment effect, the better.
The scattered solution of the Lisi Panlan mouth is accurately administered for the accuracy of the weight of the small body. According to statistics, the proportion of patients with SMA is the highest (1-11 months). The highest proportion of patients (51%). For medical insurance in SMA patients, especially children, it will provide them with hope of improving their quality of life, extending their lives, and allow patients and families to be normal in time. Integrate into society and create value.
With the continuous expansion of drug protection, the level of protection of rare disease patients has increased significantly. In addition to participating in basic medical insurance, the solution channels for medical assistance in poor patients with poor diseases are also continuously improved. In addition, according to the latest news, the US Drug Administration (FDA) has recently expanded new indications for dispersed solutions for Lisi Panlan mouth, approved to treat babies with SMA with SMA less than 2 months. The further reduction of the treatment age can see that the medicine has good safety and effectiveness, which can greatly improve the long -term survival status and quality of life of infant patients, meet the requirements of drug economy, and have good socio -economic cost advantages.
For SMA patients, the most economical and effective way is to intervene and treat as soon as possible. The Lispan mouth is administered for the staircase dose. The younger the patient's medication is, the lower the treatment burden. The sooner the better, the better the prognosis effect, and the greatest economic advantage can be obtained.
Edit: Yu Chenglin
- END -
Eat melatonin if you can't sleep?Expert: It is not recommended to use it casually
In recent years, melatonin -related products have been respected as dormant drugs by young people, especially white -collar workers,, especially white -collar workers. In the ranking of the sales vo
Love has repeatedly failed and cannot be integrated into the group ... Sleep decompression training camps heal the soul for them

Wuhan Evening News June 24 (Reporter Mao Yin Correspondent Fu Yulin) I was stunned...