Use Ai Guard to continue the dawn of life | "Ailiblin+target free" research results are released.
Author:Cancer Channel of the Medical Time:2022.07.07
*For medical professionals for reading reference
Professor Liu Jieqiong takes you to explain the value of the joint research value of Eliblin+Apatinib+Karel Pearl Mipida!
Three -negative breast cancer (TNBC) as the worst breast cancer subtype of the prognosis, compared with other subtype breast cancer, lacks clear treatment targets, has strong tumor invasion, and is often accompanied by internal organs. Once the patient steps into the advanced stage, the effective treatment plan is limited, the total survival time is short, and more effective treatment plans are needed clinically.
On May 31, 2022, the top international journal Nature Communications published an inquiry of Elblin+Carrizumab+Apatinib "Apartinib" led by Academician Song Erwei and Professor Liu Jieqiong of Sun Yat -Sen Memorial Hospital of Sun Yat -sen University. Liblin+target free "combination of three-combined treatment of advanced three-negative breast cancer patients with severe menstrual treatment, which brings" E-Base (based on Eliblin) "solution for clinical workers The latest data.
The medical community invited Professor Liu Jieqiong to share the research and clinical significance of the research on the research, helping patients with breast cancer live better and live longer.
Choose high -efficiency and low toxic combined chemotherapy partners,
Need to break through the previous "target free" insufficient
Professor Liu Jieqiong said: "So far, immunotherapy can still not fully meet the treatment needs of TNBC. Studies in Phase III have shown that the first-line treatment of immune combined chemotherapy can extend the survival of PD-L1 positive TNBC patients. Tourist treatment solutions based on immunotherapy do not bring survival benefits to patients. "
At the same time, previous studies have shown that the selection and benefits of TNBC second -line and backline are limited, and the efficacy of single drugs and combined chemotherapy is not ideal. The new TROP-2 antibody puppet drug (ADC) provides new treatment options for TNBC, but the median no progressive survival (PFS) of Trop-2 ADC is only 5.6 months. Therefore, the second-line and backline treatment of the late TNBC still has unsatisfactory treatment needs, and clinical workers have also begun immune-related exploration [2-3].
Professor Liu Jieqiong talked: "Unfortunately, in the second-line and backline treatment of advanced TNBC, related explorations of Keynote-119 and other studies have obtained negative results [4]. Research, and published related studies on the Clinical Cancer Research in 2022. The results showed that because anti -vascular production therapy can improve the immune micro -environment, immune combined with antibodium production therapy has a synergistic effect, igniting the immunotherapy TNBC TNBC New Hope [5]. On this basis, we found in Phase II research that PD-1 monoclonal anti-Corrizumab combined with anti-vascular generic drugs Apatinib's 'de-chemotherapy' combination It is equivalent to 43.3%of the patients with advanced TNBC treatment of 2 -line therapy, but the patient's median PFS is only 3.7 months. ORR benefits have not been converted into the survival benefits of patients [6]. Therefore, we think there is any It is necessary to further combine chemotherapy on the basis of immune+antiovascular generation plan. "
Professor Liu Jieqiong further talked: "In terms of the choice of chemotherapy drugs, 'high -efficiency and low toxicity' is the standard for the selection of combined therapy drugs. At present, there are many large -scale III stages of research. While significantly extended the OS of the general population, it also has considerable effects on TNBC patients.
As a new generation of microtubucta inhibitors, Ailiblin has a unique mechanism for re -painting tumor vascular reshaping, which can improve tumor blood irrigation, improve the tissue penetration of antitumor drugs, and achieve improvement of immune micro -environment and collaborative increase with other drugs. Effect. At the same time, Ailblin's perception of adverse reactions is low, the quality of life has a small impact on patients, and can ensure the tolerance of patients' medication. Based on these two major characteristics, Elibalin is more suitable for joint treatment, and based on the efficacy and safety advantages shown in the joint research of Ericbolin, we believe that Ericblin+Apatinib+Karrili Lili Lili Lili Lili Lili Lili Lili Lilly The "Ericiblin+target free" treatment mode of Zhu Mipu's combination can make patients get more benefits [7-11]. "Ailibarin joined the" target free "combination and efficacy
Realizing breakthroughs, significantly improved the survival of patients with severe menstruation
Regarding the patient's baseline characteristics, Professor Liu Jieqiong introduced: "The patient's tumor has a large tumor load in this study, and the number of median treatment lines in patients is generally experienced. 17.4%of patients used to use immune combined chemotherapy in the past. In addition, more than 40%of patients had liver metastasis. In addition, a large part of the patients in this study were less than 6 months. Impassion-130 and Keynote-355 have not been admitted to patients with less than 6 months.
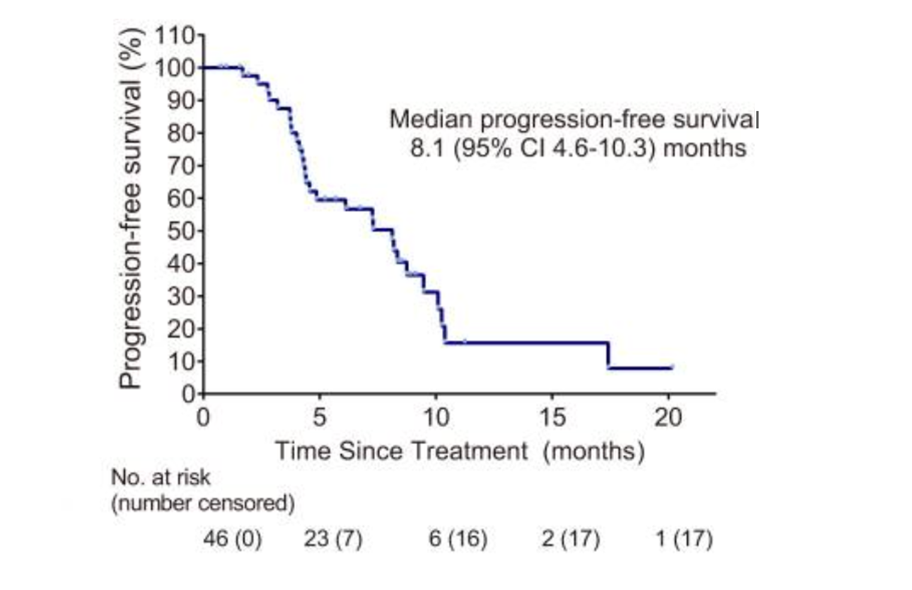
Among the patients with poor baseline conditions, the "Ailiblin+" solution has still achieved joy. Research data show that after further introduction of Ailblin on the basis of immune+anti -vascular "target free" treatment, the efficacy of joint treatment has been improved. Among the crowd (46) in the care of the treatment (ITT), Erblin+Apatinib+Karrilizab's "Eliprin+target free" combination is 37.0%, and the disease control rate (DCR ) 87.0%. In terms of survival data, the median PFS of the "Ericiblin+target free" combination for patients is 8.1 months, and the median OS has not yet reached. In terms of safety, the incidence of adverse reactions (TRAE) in level 3/4 is only 41.3%, mainly for preventable and controllable neutropenis, thrombocytopenia, etc. The toxicity occurs, and tolerance is better [2]. "
"Ailiblin+Target Free" scheme PFS data
"Ailibarin+target free" solution security data

Professor Liu Jieqiong said: "After adding Ailblin on the basis of the" target free "combination, the survival of the patient has been significantly improved (the median PFS is 8.1 months and 3.7 months, respectively). The disease relief is truly transformed into a patient's practical survival benefit. "
Regardless of the expression of PD-L1
Benefit under the "Ailiblin+target free" solution
Professor Liu Jieqiong talked: "An excellent study also needs complete biomarker analysis on the basis of efficacy and security data. On the basis of the effective effect of Liblin+target free 'scheme, tumor logo related analysis was performed. Among them, the analysis of PD-L1 expression states showed that there is no association between the PD-L1 CPS score and efficacy, regardless of the patient PD-L1 How can the expression level (positive or negative) benefit from the "Ailiblin+target" scheme. "
In addition, a number of previous studies published in Nature suggest that the three -level lymph structure (TLS) is related to the efficacy of immunotherapy. This study also conducted TLS -related analysis, and the results showed that more TLS (average area large area ≥30000 μm) ORRs were significantly higher than that of TLS (71.4% vs 25.0%) with less than TLS. Although the Asian group analysis also found that the patient's TLS and PFS are not significantly related, the association between TLS and ORR still indicates that more patients with TLS may benefit more from the "Ericblin+target free" scheme [2].
Professor Liu Jieqiong talked: "Generally speaking, in this study, a more detailed biomarker analysis has been conducted, and the beneficiaries of the" Ericblin+target free 'treatment are further clarified. The concept of 'and' individualized diagnosis and treatment '. "
Medical insurance help, exploration more than,
The "Ailiblin+target free" plan hopes to benefit more patients
Professor Liu Jieqiong concluded: "Although this study belongs to Phase II research, it is of great significance for the field of late TNBC treatment in my country and the world. In terms of value, the longest of all related forward -looking research, its 8.1 months of median PFS data is far greater than the corresponding values in previous studies. Although the data in different studies cannot be directly compared, this study still proves '' '' The efficacy of Ailiblin+target free 'scheme in advanced TNBC backline is considerable, and the toxicity of the "Ailiblin+target free" solution is relatively low.
At present, Ailibarin has been included in medical insurance, so the "Ailibarin+target" combination is still highly available among patients with limited economic conditions, which can benefit more patients. In addition, this study fully demonstrates the potential of the E-Base solution. I hope that in the future, more clinical workers can explore the efficacy and safety of Ailblin and various drugs in various molecular types. The application scenario is enriched to the application basis of Ailblin, which brings practical benefits to patients with breast cancer. "Professor Liu Jieqiong said:" The advent of such a very meaningful study is inseparable from the strong support and cooperation of many parties. Thanks to Academician Song Erwei's strong support, and thanks to all patients and their families of this study. "
Expert Introduction
Academician Song Erwei
Academician of the Chinese Academy of Sciences, chief physician, doctoral supervisor, subject leader

Dean of Sun Yat -sen Medical College of Sun Yat -sen University.
Dean of Sun Yixian Memorial Hospital of Sun Yat -sen University
Dean of Yixian Breast Tumor Hospital
Deputy Chairman of the Breast Cancer Professional Committee of China Anti -Cancer Association
Chairman of the China Clinical Oncology Society (CSCO) Breast Cancer Expert Committee
Deputy Chairman of the Breast Physician Committee of the Chinese Medical Association Surgeon Branch
Deputy Chairman of the Cancer Transfer Professional Committee of China Anti -Cancer Association
Deputy Chairman of the Guangdong Medical Association Surgery Branch
He Liang He Li's Science and Technology Innovation Award, National Health Family Planning has outstanding contributions to young and young experts, an outstanding professor of the American Chinese Medical Foundation (CMB), the country's first batch of young and middle -aged scientific and technological innovation leaders, and outstanding scientific and technological workers in the country.
He is good at the treatment of breast cancer, including the treatment of breast slicing surgery; the auxiliary chemical therapy, endocrine therapy, and comprehensive treatment of advanced breast cancer for breast cancer. Mainly engaged in early diagnosis of breast cancer, including the early diagnosis of BRCA1 and BRCA2 gene mutations to family breast cancer and serum protein fingerprint diagram, as well as minimally invasive therapy and biological treatment of breast cancer, including RNAF disturbance therapy.
Expert Introduction
Professor Liu Jieqiong
Associate Professor of Breast Surgery of Sun Yixian Memorial Hospital of Sun Yat -sen University

Deputy Director of Diagnostic Diagnosis Department of Sun Yixian Memorial Hospital of Sun Yat -sen University
Doctoral supervisor (proposed) outstanding young medical talents in Guangdong Province
2017 CSCO "35 UNDER 35" is the most potential tumor doctor
Youth Member of the Tumor Branch of the Chinese Medical Association
CSCO Transformation Medical Committee member
Young Scholar Scholar of Breast Cancer Professional Committee of China Anti -Cancer Association
Member of the Sports Tumorology Group of the China Rehabilitation Medical Association to restore the Surgery Specialty Committee
Member of the Cancer Special Committee of the Chinese Female Physician Association
2008-2010 Harvard University Massachusetts General Hospital Joint Training Dr.
Postdoctoral Poli-doctoral Center of John Hopkins Hospital from 2014-2015
Published 31 SCI papers in PNAS, CCR, JITC, NC and other magazines with the first or communication authors, and presided over 9 scientific research funds
references:
[1]. National Compirensive Cancer Network. NCCN Clinical Practice Guidelits in onCology (Breast Cancer) .Version 4.2022 - june 21, 2022.
[2]. LIU J, Wang Y, Tian Z, Lin Y, Li H, Zhu Z, Li Q, SUNG y, jia w, yang y, xu s, yao h, jiang w, song e. multicenter Phase II TRIAL of Camrelizumab Combined with APATINIB and ERIIBULIN in Heavily Pretreated Patients with Advanced Triple-Negative Breast Cancer. 2022 May 31: 301111111111111111111111111111111111111111111OREA
[3]. Bardia A, Hurvitz Sa, Tolaney SM, et al. SACITUZUMAB GOVITECAN in Metastatic Triple-Negative Breast Cancer. N English. 2021 APR 22; 384 (16): 1529-1541.
[4]. Winer EP, Lipatov O, Im SA, et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021 Mar 4. [5]. Li Q, Wang Y, JIA W, et al. Low-Dose -Agiogenic Therapy Sensitizes Breast Cancer to PD-BLOCKADE. CLIN CARINCER Res. 2020 APR 1; 26 (7): 1712- 1724. Doi: 10.1158/1078-0432.CR-19-2179.
[6]. Liu J, Liu Q, Li Y,et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: an open-label phase II trial. J Immunother Cancer. 2020 May;8(1 ): E000696.
[7]. Kaufman PA, Awada A, Twelves C, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015 Feb 20; 33 (6): 594-601.
[8]. Wu Y, Wang Q, Zhang J, et al. Incidence of peripheral neuropathy associated with eribulin mesylate versus vinorelbine in patients with metastatic breast cancer: sub-group analysis of a randomized phase III study. Support Care Cancer. 2020 Aug ; 28 (8): 3819-3829.
[9]. Cortes J,O'Shaughnessy J,Loesch D,et al.Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer(EMBRACE):a phase 3 open-label randomised study[J].Lancet.2011 ; 377 (9769): 914-23.
[10]. Kaufman, P. A.et al.Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane.J Clin Oncol(33): 594-601 Then, then, then
[11]. Yuan P, et al. Ericin Mesilaate VINORELBINE in WOMEN With Locally Recurrent or Metastatic Breast Cancer: A Randomised Clinical Trial.Medical sources provide scientific information, which does not represent the point of view of this platform


- END -
Today at 15:00, the medical science science live broadcast

Medical and Heart is a live broadcast section of medical online science populariza...
my country's scientist team reveals the entire picture of the new H5N1 bird flu virus evolution
The reporter learned from the Harbin Veterinary Research Institute of the Chinese ...