[2022 ASCO] Federation of Asko │ Ada Tasamine Treatment Progress Analysis
Author:Cancer Channel of the Medical Time:2022.06.13
*For medical professionals for reading reference

In 2022, the American Society of Clinical Oncology (ASCO) Annual Conference was held in the form of online and offline conferences from June 3-7 local time. As the world's largest and most authoritative clinical oncology "Olympic Conference", the ASCO Annual Meeting brings together many world -flow -influx oncology experts, and shared with the participants to discuss the current industry's most cutting -edge clinical oncology research results and tumors. New technologies. At the same time, the conference also announced a number of heavy research results in the field of urinary tumors. This article mainly shares the research progress of Aparel amine in the field of prostate cancer in the field of neo -assisted/auxiliary therapy, as well as 3 research data of Aparolymid therapy, and invites Professor Dai Bo from Fudan University Cancer Hospital for everyone to give everyone to everyone for everyone In -depth interpretation.


2022ASCO Annual Meeting Great
ATLAS Research: Evaluate local high -risk or advanced prostate cancer (HRLPC) patients with APA+ADT new assisted and auxiliary treatment effects on the III stage random dual -blind control research: the first reported patient characteristics and different radiotherapy schemes (2022 ASCO ABS 5084) [1 ]
At present, HRLPC's treatment schemes include long -term androgen deprivation of therapy (ADT) and radiotherapy, but patients still face higher risk of metastasis and death. ATLAS (NCT02531516) is an exploration of Apaamine (APA)+ADT new assisted therapy. The anemia hormone agonist (GNRHA) and standard in vitro radiotherapy (EBRT) can improve whether high -risk patients have no metastatic survival ( MFS) Study of III. The report announced the baseline characteristics distribution of the patient and the application of different radiotherapy plans.
HRLPC patients who meet the requirements of the group are Gleason scores (GS) ≥8 or 7, PSA ≥20 Ng/ml, clinical staging ≥ CT2C, ECOG PS 0/1, Charlson Common Election Index (CCI) ≤3, and according to the same according to GS, pelvic lymph node status, close -up enhanced therapy use and area are layered. Patients in the group are randomly divided into APA+GNRHA or placebo+GNRHA group. Study a total of 30 treatment cycles in the treatment, first adopt new assisted/synchronous radiotherapy+bibaramide (50 mg/d)+adt ± APA (240 mg/d), 4 cycles for treatment; continue to receive 26 cycles of ADT ± after radiotherapy after radiotherapy treatment; Apatamine 240 mg/d adjuvant treatment. The main end point is MFS (see Figure 1).
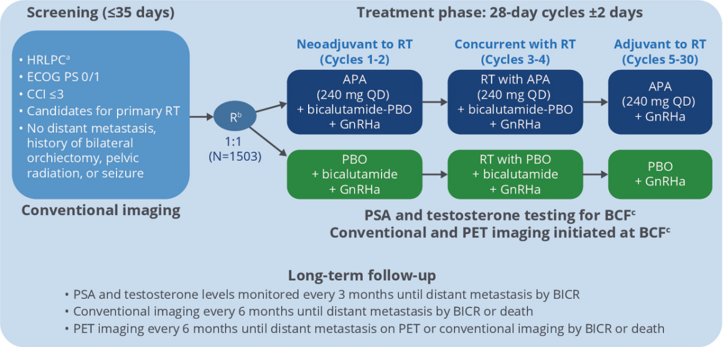
Figure 1.Tlas research design
Studies were included in a total of 1503 HRLPC patients from 266 centers from 266 centers from North America, Latin America, Europe and Asia. The median age is 67 years old, ECOG PS 0/1 is 89%/11%. The tumor grading when entering the research is as follows: high risk (66%), extremely high risk (34%). The median PSA is 6.3 ng/ml. The clinical staging is CT2 (44%), CT3 (50%), CN1 (13%) (see Figure 2).
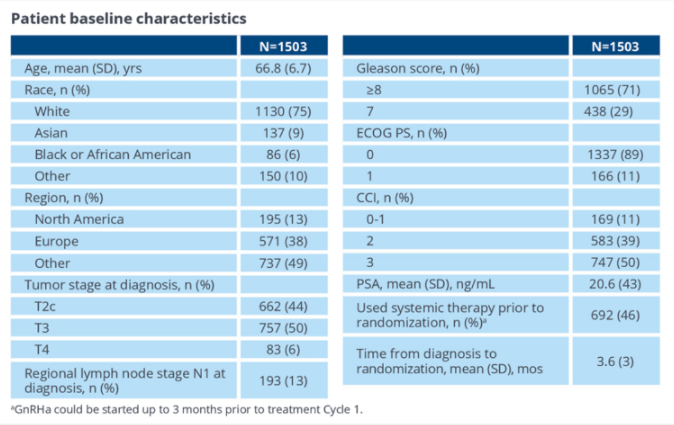
Figure 2. Patient baseline characteristics
In terms of the selection of radiotherapy schemes, 90%of patients use EBRT to treat prostate/pelvic 6-8 weeks (cumulative 78-81 gy), and 10%of patients use the latest low-segmentation radiotherapy scheme (20x3 GY/D or 28x2.5 GY /D). 6%of patients use EBRT combined with short -distance radiotherapy (Figure 3).
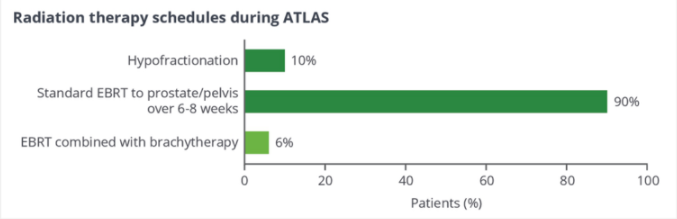
Figure 3. Radiotherapy choice
In summary, the baseline characteristics of the ATLAS research crowd reflect the situation where high -risk and high -risk patients are affected by pelvic lymph nodes in clinical practice. The application of low -segmentation radiotherapy also reflects the changes in recent clinical evidence and guidelines. In 2022, the latest "Guidelines for Clinical Limitations" released by the Annual Meeting of the Urology Association (AUA), which is recommended for high -risk prostate cancer patients suitable for EBRT prostate cancer patients with low scoring EBRT [2] (Moderate recommendation; Class of evidence: C class). For HRLPC, the combined treatment strategy of newly -assisted/auxiliary radiotherapy and new androgen receptor inhibitors (such as APA) is worth looking forward to in the future.
New Auxiliary ADT combined with Aipilong and Aparoamine to treat a random stage II study of local high -risk prostate cancer patients: pathological relief is related to PSMA imaging (2022 ASCO ABS 5085) [3]
Patients with local high -risk prostate cancer (HRLPC) still have a high risk of recurrence and metastasis after a cure prostate dehodia (RP). Therefore, it is necessary to explore the pre -operative new assisted treatment. This II phase study is the pathological relief rate of newly -assisted ADT in patients with HRLPC patients with ADT combined with AAP or AAP combined with Aipheamine. The preliminary result of the study was reported.
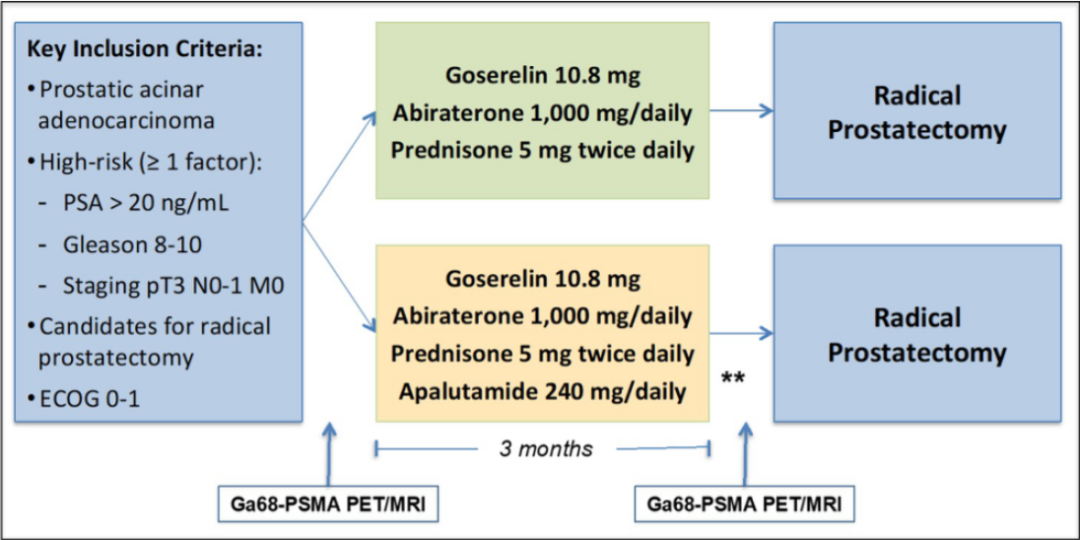
Study before patients with HRLPC (Gleason ≥ 8 and/or CT3N0-1 and/or PSA ≥20 Ng/ml) are used to radical prostate slicing (RP), use gosherelin+acetic acid acrylic acid and bomonettisone. (AAP scheme) or AAP+AAP+APA solution for three -month new assisted treatment. The main ending point is the pathological relief rate (PCR) or small residual lesions (MRDs, tumors ≤0.5 cm) (see Figure 4). The secondary end point is safety, residual tumor load rate (RCB, the number of tumor volume X cells) ≤0.25 CM3, 镓 68 prostate special -specific membrane antigen (PSMA) positive electron emission fault scan (PET)/magnetic resonance and biochemical recurrence rate (Br) correlation. Figure 4. Study design
The results of the study showed that a total of 62 patients with HRLPC were included, randomly divided into AAP+APA groups (n = 31) and AAP groups (n = 31). The median age is 65 years (range 47-77 years). In NCCN hazardous groups, high risk (19%), extremely high risk (76%), local (N1) disease accounts for 5% (79% CT3, 65% Gleason 8-10, 57% PSA ≥20 Ng/ml). In terms of PCR/MRD or RCB ≤ 0.25 CM3, there is no statistical difference between the research group.
The BR rate of patients with RCB ≤ 0.25 CM3 is 14%, while the BR rate of patients with RCB & 0.25 CM3 is 38%(p = 0.118). At present, the median follow-up time is 2.6 years. All patients with PSMA-CR and RCB ≤ 0.25cm3 (n = 11, 18%) have not renewed.
In terms of security, two patients with AAP groups had a level 5 adverse event (AES) (AES) (AES) (AES) (AES), which were pulmonary embolism and sudden death, all after surgery). Nine patients had 3-4 levels of treatment related AES, accounting for 14.5%(of which 6 cases of AAP+APA group; 3 cases of AAP group). The most common level 3-4 AES is hypertension (11.3%), elevated AST/ALT (3.2%), and rash (as shown in Figure 5).
Figure 5. Bad events occur
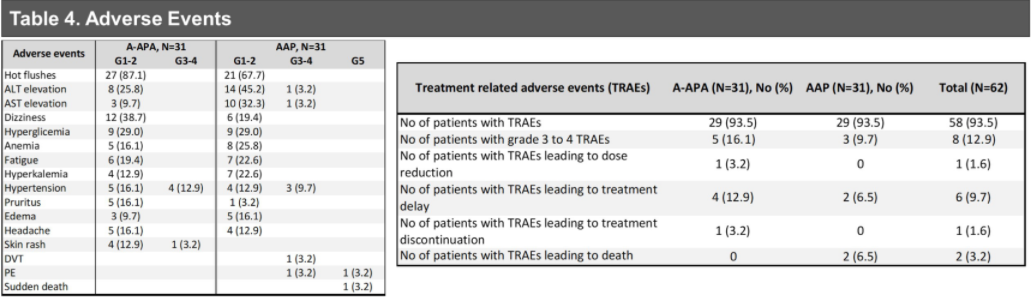
In summary, there is no significant difference in PCR or MRD between AAP+APA and AAP groups. Although there are rare cases of PCR or MRDs after AAP+APA, a considerable part of patients have achieved good pathological relief at RCB ≤ 0.25 CM3. Therefore, the PSMA-PET relief can be used as a potential pathological relief alternative indicator.
Dynamo: A sequential study of metastatic removal resistance prostate cancer (MCRPC) has been approved and promising (2022 ASCO ABS 5059) [4]
The response situation of MCRPC patients usingrogens receptor inhibitors is obviously differentiated, but due to the lack of appropriate biomarkers to identify different MCRPC sub -groups, the development of effective joint schemes for MCRPC patients is always facing difficulties. In clinical studies, a homogeneity treatment is used for heterogeneous diseases. In the past, we attributed the invasive mutant prostate cancer to a category of poor reactions to the male hormone receptor inhibitors, and observed that the use of Cabcarb joint schemes using Cabcarb can improve the prognosis of the sub -group. At the same time, some forward-looking research results show that anti-CTLA-4 treatment may be conducive to patients with androgen reactive diseases. We assume that compared with previous historical data, the combined treatment of differentiated MCRPCs based on the decline of early serum science markers (such as PSA) will improve their overall survival (OS).
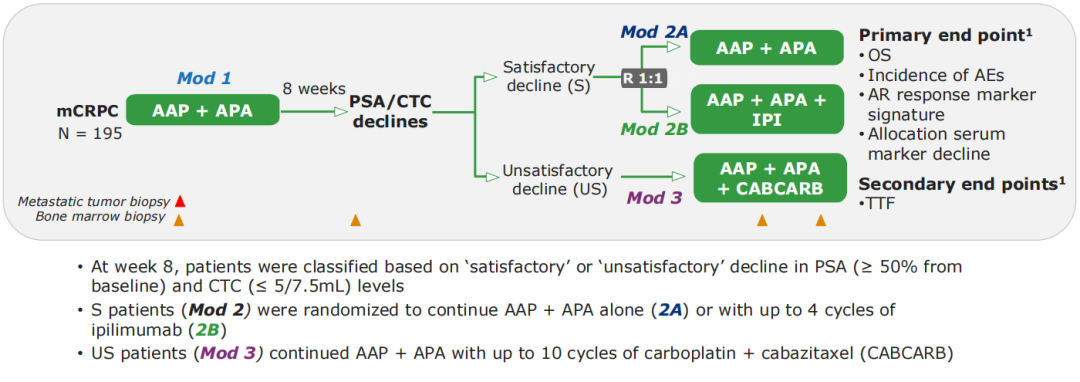
Phase II clinical research (Dynamo, NCT02703623) of this open label was included in patients with AAP+APA for patients with Aap+APA for the treatment group 1. In the 8th week, the decline in the decline in the decline of the patient's prostate cancer special antigen (PSA) and circulatory tumor cell (CTC) (PSA began to decrease from the baseline ≥50%, CTC ≤ 5/7.5ml) was divided into "compliance" (s) Or "not in line". Group S patients enter the treatment group 2, and randomly receive AAP+APA treatment (2A scheme) or AAP+APA combined with up to 4 cycles of Ipmu monoclonal anti -anti -therapy (IPI, 2B scheme). Patients in the US group entered the treatment group 3 and continued to receive the AAP+APA combination of up to 10 cycles of card platinum+Cabcarb for treatment (see Figure 1). Since then, patients have continued AAP+APA treatment until the disease progressed. Starting from the patient into the treatment group 2 or the treatment group at 3, the survival period (OS) (see Figure 6). According to historical data, the estimated median OS of the treatment group 2 and the treatment group 3 is 36 and 16 months, respectively.
Figure 6.Dynamo research design
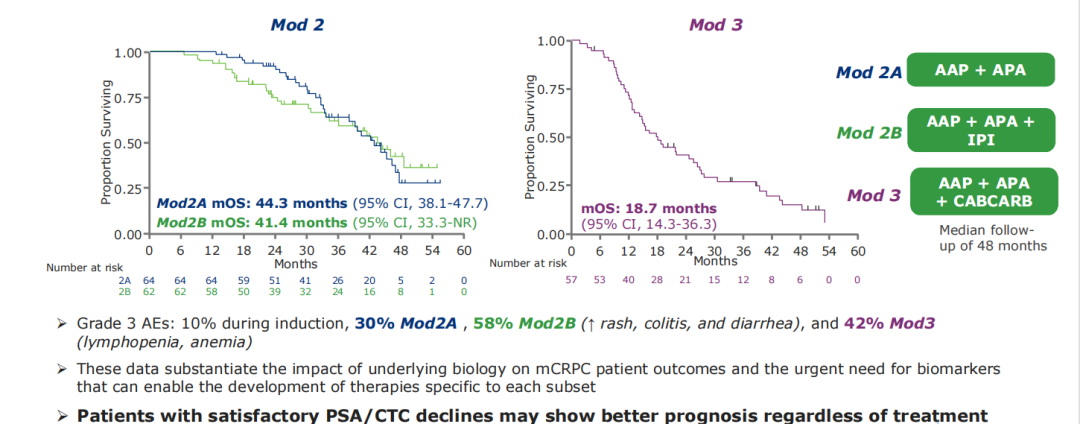
The results of the study showed that from May 2016 to August 2019, a total of 195 MCRPC patients were included. 128 (67%) patients were assigned to group S, of which 64 were randomly received for AAP+APA ± IPI treatment. Three patients withdrew from the study during the 1st period of treatment group. Another 64 patients were assigned to the US group, of which 59 were treated with Cabcarb. Compared with the S group, the new hair movement of patients in the US group (33% vs 59%, P <0.001), the RECIST standard can measure the lesion (23% vs 42%, P = 0.01) and liver metastasis P = 0.01) is more likely. The healing group 2A, 2B, and 3 serious adverse events were 3 cases (4.7%), 21 cases (33%), and 6 (10.2%). The treatment group 3 died of neutral granulocytes. The median follow-up is 48 months, the median OS of the treatment group 2A is 44.3 (95% CI 38.1-47.7) for a month, and the median OS of the treatment group 2B is 41.4 (95% CI 33.3-not reached) for a month. The median OS of 3 is 18.7 (95% CI 14.3-36.3) a month (see Figure 7). Figure 7.Dynamo research results
In summary, this study data confirms the impact of basic biology on the prognosis of patients with MCRPC and the urgent need for exploring biomarkers. The ideal biomarker is conducive to the development of treatment strategies for different CRPC people. In the future, more related studies need to be further identified to identify these biomarkers.
Expert Reviews
As we all know, the total survival (OS) is the "gold standard" of the curative effect of antitumor drugs, which is widely used in clinical research of antitumor drugs. However, with the continuous development of anti -tumor treatment, the patient's OS is continuously extended, and the acquisition of OS data requires a larger sample amount, longer follow -up time, and more funding support. Therefore, it is especially important to find short -term, convenient and effective "alternative" efficacy prediction indicators. SWOG 9346 [5], Chaarted [6] studies have confirmed that PSA is a strong prediction indicator for the benefit of survival benefits of prostate cancer patients. A sequential study of MCRPC released at the ASCO Annual Conference [4] reported that after the treatment of MCRPC patients in AAP+APA, it can be re -paid according to the different degrees of decreased PSA and CTCs to guide personalized treatment strategies (AAP+ Apa ± ipi, AAP+APA+Cabcarb). This is related to SWOG 9346 [6], Chaarted [7] has the wonderfulness of "different songs", which shows that the rapid and deep PSA decline in patients with prostate cancer has predictive value for long -term benefits.
In addition, for patients with local high -risk or later PCs, the preferred treatment plan recommended by the guide is still the combination of cure and ADT, but the benefits of patients still need to be improved. Therefore, the exploration of new assisted/auxiliary therapy in combination with APA came into being. At this meeting, the first report of the APA+ADT new assisted/auxiliary treatment of HRLPC patients (ATLAS) [1] The baseline characteristics and different radiotherapy types of the baseline reflected the baseline characteristics to a certain extent. In the case of receiving radiation therapy in clinical practice, it is also expected that the APA+ADT neo -auxiliary and auxiliary therapy can bring greater benefits to HRLPC patients. In addition, there is a new phase of Phase II research on the treatment of local high-risk prostate cancer with ADT and Aipheamine for local high-risk prostate cancer [3]. Advantage.
In the future, we look forward to the addition of APA in new assisted/auxiliary therapy plans to bring better treatment options to patients with HRLPC to help these patients achieve better clinical benefits.
Expert Introduction
Professor Dai Bo
PhD, chief physician, doctoral supervisor

Director of the Department of Urology, Fudan University Affiliated Oncology Hospital
Secretary -General of the Youth Committee of the China Anti -Cancer Association Urinary Men's Reproductive Cancer Professional Committee
Standing Committee member of the China Healthcare International Exchange Promotion Capital Mirror Insider Surgery Branch
Member of Shanghai Urology Society
He is the editorial board of the following academic journals: "Chinese Magazine of China Urology", "Chinese Cancer Magazine", "Journal of Southern Medical University", "Chinese Cancer Prevention and Control Magazine"
The two sessions of the China Anti -Cancer Association's Urinary Men's Reproductive Cancer Special Committee "Chief Minimally Invasive Surgery Doctor"
China Anti -Cancer Association's Urinary Men's Reproductive Cancer Special Committee "MDT Excellent Dr."
People's Good Doctor (Jinshan Camellia -Outstanding Contribution Award, the field of urinary tumors)
Two good doctors online "good husband of the year"
Shanghai Paths Science Society of the Year of the Year
Renxin doctor (Shanghai Outstanding Specialist Award Nomination Award)
Outstanding young talent in Shanghai Health System
Top Ten Medical Youths of Fudan University
references:
[1] .sandlerhmet al.2022 ascoabs 5084.
[2]. Easthamjaet Al.Clinical Localized PRostate Cancer: AUA/ASTROGUIDELINE (2022) [3] .bastos da et al.2022 ascoabs 5085.
[4] .viscuse pv et al. 2022 ASCO ABS 5059.
[5] .Hussain m, et al. J clin oncol. 2006: 24 (24): 3984-90.
[6]. Harshman LC, et al. J clin oncol. 2018: 36 (4): 376-382.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
What's wrong with the black line on the baby's nails?Is it tight?

The black line of the baby's nails is called nail -like black nails or longitudina...
Standard treatment of patients with tuberculosis can cure
Dahe Health News reporter Cao CongTuberculosis is a chronic infectious disease tha...