Standing on the shoulder of PD-1, the drug that leads the fourth wave of changes comes
Author:Kenji Bureau Time:2022.07.09
It has not been a class of drugs for a long time. It is lively like the recent two -specific antibodies, and many good news announced one after another.
Kangfang's Kadinili Mipide was listed on June 29 to treat patients with recurrence or metastatic cervical cancer who had previously received platinum chemotherapy failure.
It is a halo: it is both the first domestic bilateral specific antibody and the world's first PD-1/CTLA-4 dual anti.
On July 5th, Kadinili surveyed the first batch of products to ship, and the price and patient aid plan were also released: the average annual cost of not exceeding 198,000 yuan. In addition, its listing time has caught up with this year's medical insurance negotiation declaration, which may be included in medical insurance during the year.
Roche also launched a dual anti -drug: On June 8th, the world's first CD20/CD3 T cell combined with double -specific anti -LUNSUMIO was approved to be listed in EU conditions.
In addition to the two double -resistance listing in one month, there are also large acquisitions. On June 23, a wholly-owned subsidiary of Chinese Biopharmaceuticals announced that it plans to acquire all the issued ordinary shares of the Nasdaq listed company F-Star at a price of $ 161 million. F-Star is a dual-resistant/multi-anti-development enterprise, and its core barriers are a dual anti-technology platform.
In addition, the recent IPO of the Science and Technology Board has received Zhixiang Jintai, which is accepted by the Shanghai Stock Exchange, mainly in the research products that also have a double-specific antibody drug. Essence
Double resistance is unprecedented.
The darling of the post-PD-1 era
Coupled with Kangfang Bio's Kadinilicab, there are 6 types of special antibody drugs on the world, and a Catumaxomab was delisted in 2017. These dual -resistant treatment areas are mainly tumor directions.
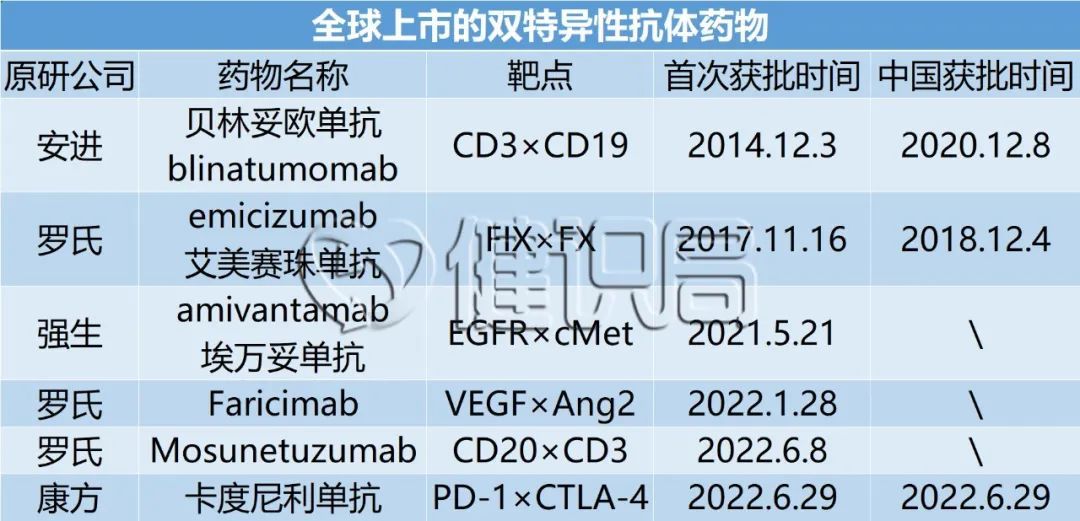
In 2020, Raymond Deshaies, an academician of the American Academy of Sciences, posted in the Nature magazine that bilateral antibodies led the fourth pharmaceutical revolution.
The report of the Chen Xiaoyuan team of Tsinghua University in the Nature Reviews Drug Discovery report can also be seen that the emphasis on dual resistance in the industry is also available in the industry: as of July 2021, in the next generation of tumor drug pipelines, cell therapy ranks first, followed by bi -specific antibodies and multi -multi -multi -multiple Specific antibodies, the two have become drugs that dominate the FIC pipeline in the field of tumor.
In June of this year, at the ASCO Annual Conference of the Tumor Field, there was a special topic "Bispecifics: Are Two Better than One?" This is the first time in ASCO's history to discuss three bilateral antibodies research.
These three double resistances are Astrikon's PD-1/CTLA-4 dual-resistant Medi5752, Merus's HER2/HER3 dual anti-Zenocutuzumab, and Kangfang creature's Kadinilicab.
China's bispentine antibody drugs have started late, but they are quite lively. Hengrui Medicine's SHR-1701 and Kangning Jerry's KN046 followed Kang Fang, which has been in the third phase of the clinical stage.
In addition, a number of pharmaceutical companies such as Cinda Biological, Baiji Shenzhou, Jiahe Biological, Beida Pharmaceutical, Zhengda Tianqing, Junshi Biological, Tianguang Shi, Platinum Medicine, Jiannenglong Bio, etc. are still chasing me. Following PD-1, short soldiers in the field of double resistance are connected.
Since 2018, investment and cooperation development in the field of bilateral -specific antibodies has been significantly active. With the listing of Kangfang Bio's Kadonilies, double anti -drugs will usher in the first wave of listing, starting the stage of commercial competition, and its development potential is huge.
Transnational pharmaceutical companies are also gathered here. Roche, Anjin, Pfizer, Sanofi, and Lili have established a bispectonic antibody R & D technology platform. Some of the bispentine antibodies on some corporate pipelines have exceeded 10 models.
Overall, this time, Chinese pharmaceutical companies are not too slow. The smoke of PD-1 is still filled, and the dual resistance of local pharmaceutical companies will compete with the cross-border giant. What kind of thrilling will be staged?
Where is the charm?
The concept of bispecific antibodies first appeared in 1960, and it was not until decades after clinical practice.
"Double resistance" is actually not corresponding to "monoclonal anti -anti -resistance". "Monoclonal antibody" is a monoclonal antibody, which refers to the use of monoclonal technology to produce antibodies. The previous monoclonal antibodies are generally aimed at a target, such as PD-1 and ALK, so monoclonal antibody is understood as "single target antibody". Single cloning technology can also produce antibodies that are also targeted at two targets, namely "bilateral antibodies", which are referred to as "dual antibodies" in Chinese.
From the perspective of the mechanism of action, dual resistance is mainly divided into 4 categories: cell bridge, dual -target blocking, immune cell activation, and protein composite generation. The industry believes that double resistance is expected to fill the indication blank of some small molecular drugs and monoclonal anti -anti -anti -anti -drug drugs.
Dual resistance can connect T cells that kill cancer cells. One end is combined with cancer cells, and one end and T cells are combined to pull T cells to cancer cells, which greatly improves killing efficiency. Double resistance can also pull the proteases and substrates related to the treatment, to achieve the treatment effect.

A good antibody with good performance and security is better than a single target. Researchers at a tumor research institute believe that: "The new structure brings a new mechanism, and the dual -characteristic antibody expands the possibility of monoclonal resistance."
At present, there are enough realistic case support this to be argued:
Roche's Vegf/ANGPT2 dual-resistant Faricimab has solved the problem of Abercip's medicinal interval, and the potential effect is better; Johnson & Johnson's EGFR/C-MET dual-resistant AMIVANTAMAB solves the problem of EGFR EX20Ins mutations and EGFR classic mutations resistance. It
Kangfang's PD-1/VEGF dual anti-AK1112 broke through the risk of severe bleeding that Beduzab may face in the scale NSCLC.
During the ten years of 2011-2021, 308 dual-resistance related research was conducted worldwide, involving 126 drugs, and more than 93.5%of it was in stage I or II.
Double resistance is a good thing, but it is far from reaching the point of outbreak and crowded. A consensus in the global pharmaceutical industry is that it is not easy to develop dual resistance. In May 2021, the FDA issued the Guide to the R & D Project of Both Specific Antibodies, which reminded pharmaceutical companies that the development challenges of dual anti -anti -development.
The law of new drug research and development is also confirmed in both resistance: high investment, high risk, failed, continuously overturned. The world's first dual anti -Kagoso monoclonal anti -anti -anti -anti -anti -anti -resistance was even tragically delisted.
Double resistance golden age
Kangfang Bio has successfully launched the first domestic double resistance, which has been 13 years since the world's first double resistance listing. This is also the layout of the giants, failed, tasted success, you chase me, and you have a lot of money.
In 2009, Cado Monopoly was born to treat malignant ascites. This is the world's first bi -specific antibody, jointly developed by Trion Pharma and Farsonus.
The Cado Mupage has a frightening destiny: due to its large side effects and other business factors, it has been discontinued in 2017 and has been discontinued in 2017.
At that time, targeted drugs and immunotherapy were in full swing, and PD-1 would not be available for several years. The concept of double-specific antibodies was too advanced for the pharmaceutical market at that time.
After the Cado monoclonal resistance, the second double resistance was Anjin's Belinso monoclonal resistance. It was listed in December 2014. It is mainly used to treat leukemia. It is also not sold well. The sales in 2021 are less than 500 million Dollar.
In terms of sales performance, only Roche's hemophilic drug Emmy Saizhu's single compression can be dazzling, surpassing the threshold for heavy drugs, sales of sales exceeded $ 2 billion in 2020, and exceeded 3 billion US dollars in 2021, which has become in recent years. One of Roche's most important new medicine.
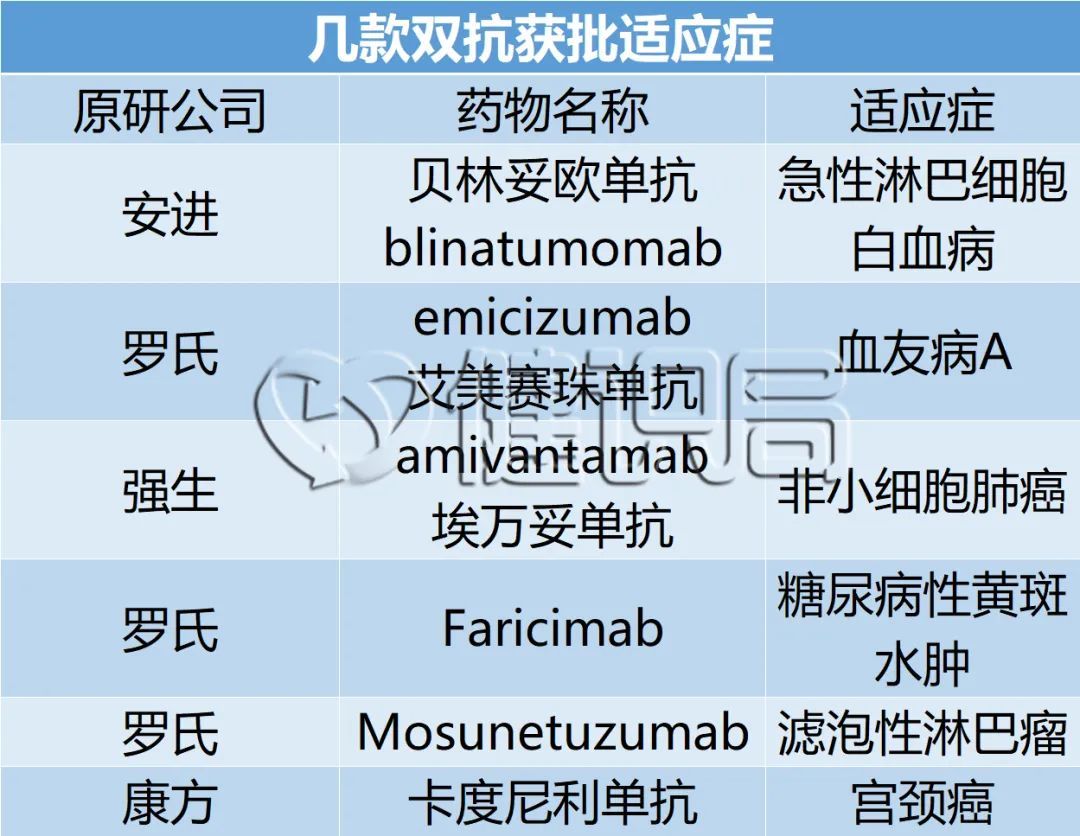
However, the situation of Emmy Saizab's resistance in China is also a little embarrassed. In November 2018, Emmy Saizabu was approved in China as a clinical urgent need for medication. According to calculations, patients with 60 kg of patients with Emmy Sai Zhuzhuo have reached 1.2 million yuan.
Guan Tao, the person in charge of the Rare Disease Love Center of Beijing House, told Jianzhi Bureau: "The effect of this medicine is very good, it is completely disruptive, but there is no progress in medical insurance."
The double anti -market may open in the future. Some institutions predict that the global market size of dual anti -drugs will exceed 50 billion yuan in 2025; the size of the Chinese market is expected to increase to 5 billion yuan by 2024.
In April 2022, CDE first released the technical guidance principles for Double Anti -Development, which explained the aspects of clinical trial risk control, best administration strategy, clinical trial design, immunogenicity, and biomarker development.
The golden age of double anti -drug seems to be here.
Writing | Zhang Ling
Edit | Jiang Yun Jia Ting
Operation | Valley
Illustration | Visual China
- END -
Jinan Earthquake Popularization Museum resumed announcement
Dear citizen friends:In order to give full play to the functions of the popular science museum and enhance the awareness of the public earthquake prevention and disaster reduction, combined with the c
Situation of Hebei New Crown pneumonia on June 6

June 6, 2022 At 0-24:00 on June 6, 2022, there were no new local new...