Production in 1 day, 8 days back!The first "fast" CAR-T human clinical trial results were officially released
Author:Cancer Channel of the Medical Time:2022.07.12
*For medical professionals for reading reference
"Racing" with the Death, shorten the CAR-T manufacturing time from 2 weeks to 1 day, greatly reducing the cost of manufacturing and shortening the waiting time of patients-this "economical application" breakthrough technology is worth looking forward to!
CAR-T therapy is a revolutionary technology in the field of tumor today. For patients with recurrence and difficulty in treating acute B lymphocyte leukemia (R/R B-ALL), CD19 targeted CAR-T therapy, the complete relief rate can reach 67% -93%.
However, traditional CAR-T technology usually requires 9-14 days of cell production time. Patients often need 3-4 weeks to wait until CAR-T cells are lost after receiving blood collection. The long waiting time may cause the patient's condition to intensify, and it also increases the treatment cost, and the treatment burden of obviously aggravated.
So, is it possible to reduce the in vitro preparation time of CAR-T cells on the basis of ensuring efficacy and safety, return cells as soon as possible to make patients benefit as soon as possible?
"Four years ago, when we discussed with R & D partners, we mentioned this clinical demand." Professor Zhang Xi, Department of Hematology of the Second Affiliated Hospital of the Army Military Medical University (Chongqing Xinqiao Hospital).
"At that time, our domestic patients chose the time of CAR-T therapy, which was often selected to implement it at the last moment when the disease was treated. At the time, the patient's leukemia cells have a high load and have an impact on curative effects and safety. In addition, the patient is likely to lose the opportunity to treat in the process of waiting. Therefore, we started from the starting point of solving this practical clinical problem and launched a new CAR- The research and development of T technology. "
Therefore, with the team of Professor Zhang Xi and related companies, the basic research of the new CAR-T technology-the FastCar platform. After a series of technological innovations, pre-clinical studies have found that the CAR-T cell GC007F produced under the new FastCar platform has not only shortened the cultivation time to 24 hours. It can be used for clinical application 8 days after blood collection. The ability is stronger than conventional CAR-T cells, less cells consume, and longer in the body.
Based on the results of pre-clinical research in the early stage, in 2019, Professor Zhang Xi team initiated a multi-center I phase I intervention clinical research (registration number: ChicTR1900023212): Discuss the safety and recurrence of recurrence of FastCAR-T cells, the safety and recurrence of recurrence of CD19+acute lymphocyte leukemia disease and Effectiveness.
On June 24, 2022, the research results were officially published in the Blood Cancer Journal of Hematology Specialty. The article shows that the first batch of 18 patients with recurrence of recurrent lymphocyte leukemia who received the first batch of Fastcar-T cell therapy and found that the therapy was that the therapy was Not only is it safe and controllable, but its cells are more "young" compared to traditional CAR-T. They have stronger expansion capabilities in the body, long retention, and good curative effects. It is worth further research and exploration.

Figure: Research and publishing information
After the article was published, the "Medicine Blood Channel" talked about Professor Zhang Xi for the first time and asked Professor Zhang to explain the relevant background and clinical significance of this study.

Exploration: What is Fastcar-T
Generally, the traditional CAR-T pre-preparation process includes the separation, activation, transfection, and amplification of T cells, and the FastCAR-T related technology platforms can quickly prepare and rectify the transfection at the same time, which will reduce the preparation cycle to less than 24 hours. Based on this technology, it is more likely to develop younger CAR-T cell products that are younger, less depleted, and better killed.
According to Professor Zhang Xi, preclinical studies have confirmed that the Fastcar-T platform does have the above potential superiority compared to the traditional CAR-T platform, including: including:
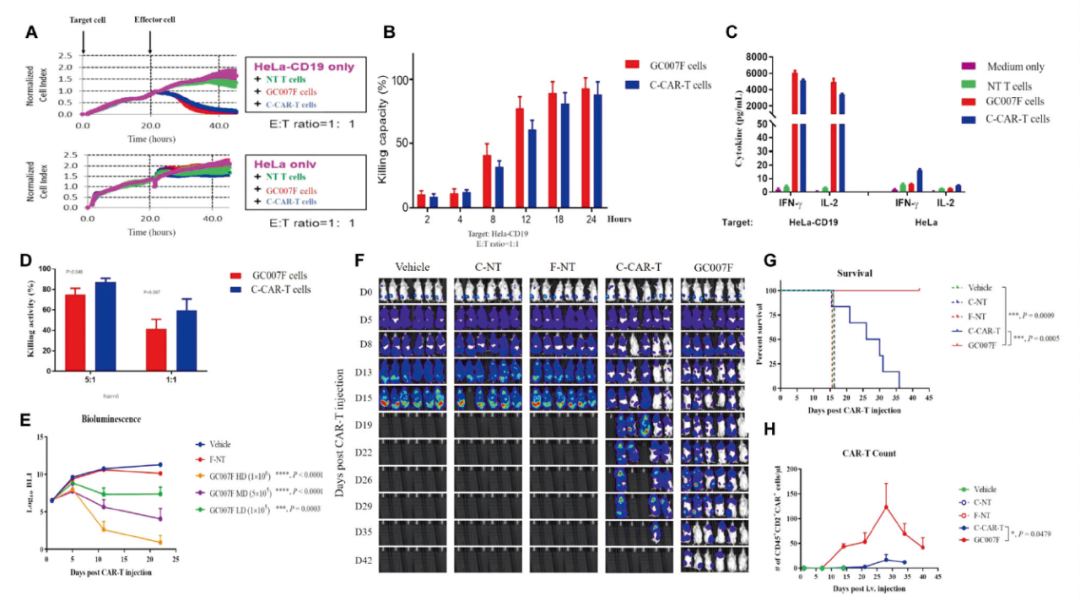
1. G007F cells are more "young": streaming cell analysis shows that GC007F cells have a higher proportion of central memory T cells (TCM), and the proportion of effect memory T cells (TEM) is lower, so it is prompted that GC007F cells are in Earlier differentiation, the cell table type is younger. Figure: GC007F cell characteristics (from the original)

2. The in vitro proliferation capacity of G007F is stronger: After being trained with CD19+tumor cells, it will activate GC007F Car-T cells and release IL-2, IFN-γ and other cytokines. CAR-T has a stronger in vitro tumor killing effect.
3. GC007F's in vivo tumor killing ability is excellent and reduced cell depletion: Animal test shows that GC007F has a significant inhibitory effect on RAJI cells in mice. The curative effect is similar or even better than conventional CAR-T cells. In addition, its retention time in the body has reached at least 40 days, showing less cellular exhaust levels.
Figure: GC007F cells and in vivo tumor killing ability (from the original)
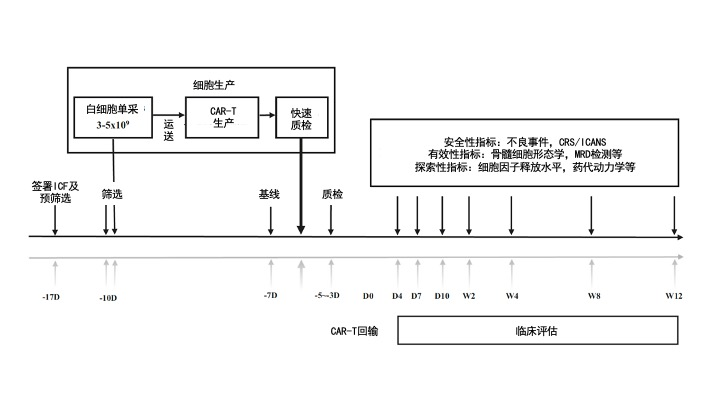
Based on the results of the above clinical study, Professor Zhang Xi team combined with 7 hospitals in China to screen for 21 patients with recurrence or refractory acute B lymphocyte leukemia (seeing the decline in the group standard) for the first time. test.
First of all, the patient will perform peripheral blood single nuclear cell collection (3-5 × 109), and then after 24 hours of production and in vitro quality testing, the CAR-T product GC007F can be returned to the patient's body 8 days later. Studies are divided into 3 dose groups according to the concentration of CAR-T cells: 0.5 × 105, 1.0 × 105, 1.5 × 105CAR+T cell/kg. In the end, a total of 18 patients entered the efficacy assessment.
准 Patients' enrollment standards:
(1) Men or women, age 14-70 years;
(2) Clarify the diagnosis as recurrence or refractory acute acute B lymphocyte leukemia (B-ALL):
a) Constraction: After 2 or more bone marrow recurrence or album hematopoietic stem cell transplantation (Allo-HSCT), the bone marrow recurrence, the proportion of primitive/naive lymphocytes in the bone marrow ≥5%;
b) Repair: The CR state does not reach the CR state after 2 rounds of standard chemotherapy;
c) Patients with Patients positive (PH+) B-ALL in Philadelphia must meet any of the following: i. Tyidase kinase inhibitor (TKI) intolerance; II. First-line, second-line TKI did not progress after treatment; III. Not suitable for Allo. Not suitable -HSCT;
(3) ECOG ≤ 1;
(4) The estimated survival period is ≥3 months;
(5) Pinded organ function reserves.
Figure: clinical research framework diagram (from the original text, translation of the medical community)
个 The research results are divided into 4 dimensions:
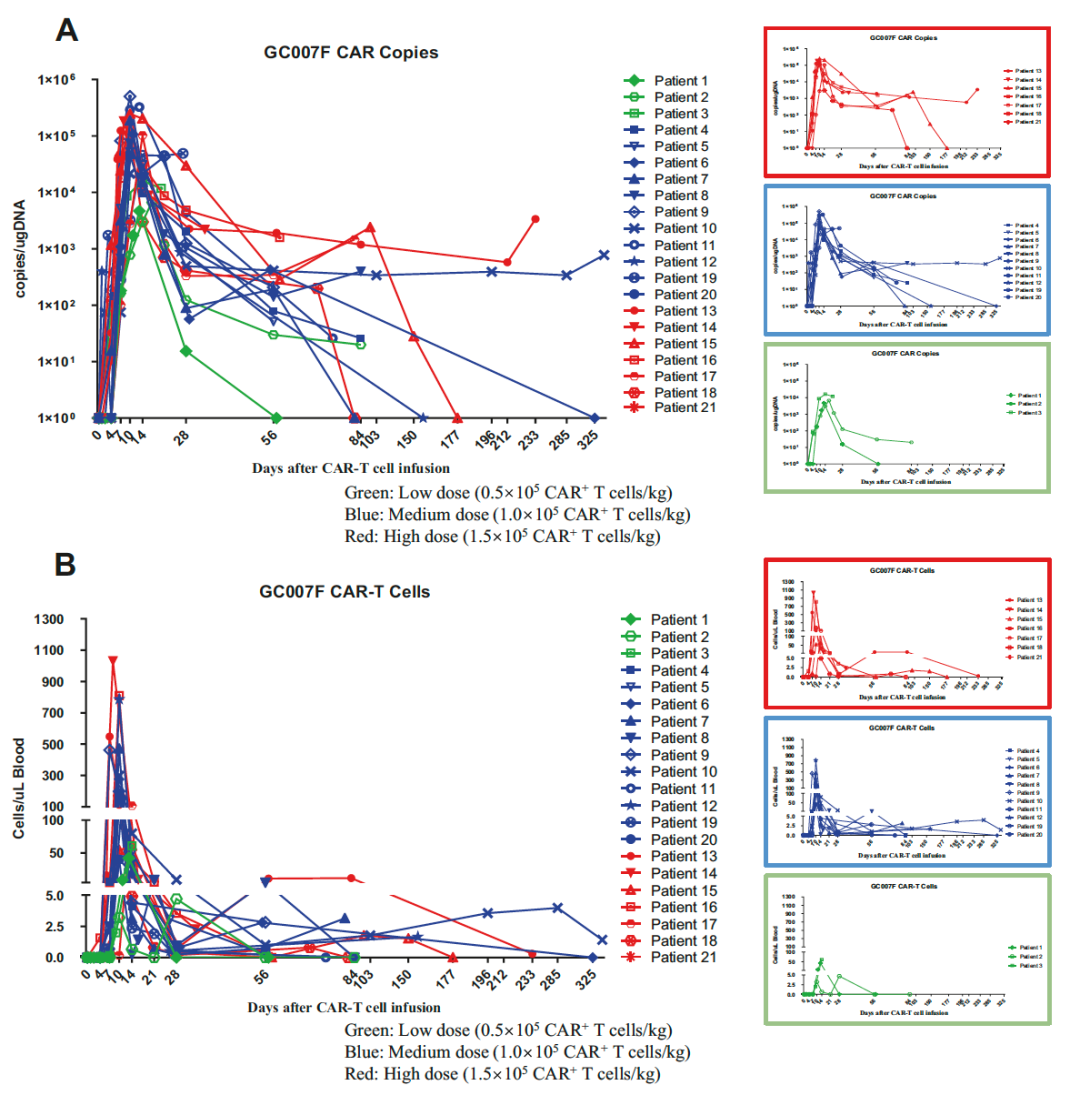
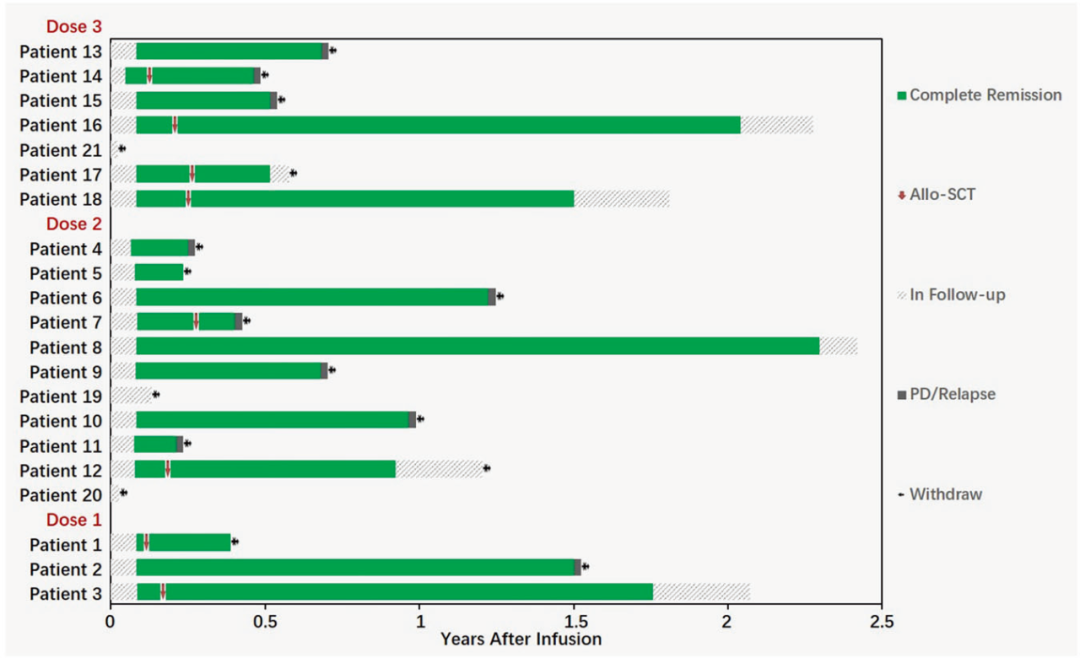
1. Cell retention: The concentration of CAR-T cells reaches its peak on the 10th day (the average DNA replication number is 104899.5/μg), and the median duration is 56 days. The longest duration is 11.7 months and the patient Still followed.
Figure: CAR-T cells detected by QPCR and streaming cell meters at 21 patients with peripheral blood (from the original)
2. Condition relief: Patients with 100%of patients in 28 days after CAR-T are completely relieved (CR), and 94.4%reaches a small residual lesion (MRD) negative. 83.3%of patients in CAR-T had reached CR 3 months after infusion, and 77.8%reached MRD negative. The maximum relief period is 29 months (hematopoietic stem cell transplantation).
3. Poor events: The total incidence of cytokine release syndrome (CRS) is 95.2%, and the incidence of CRS in severe (≥ 3) CRS is 52.4%. The incidence of toxicity of the nervous system is 28.6%. None of the patient died of complications.
4. The ending of the patient: Among the 18 patients, eight were subsequently transplanted by autologous hematopoietic stem cells, three of which continued to maintain MRD negative, 2 cases recurred, 3 transplanted related complications, the longest -free disease survival period It is 27.3 months. One of the remaining 10 people kept MRD negative, 8 recurrence, one died of severe infection, and the longest -free survival period was 29 months.
Figure: The ending of the 18 patients who can evaluate the effect (from the original)
CAR-T fast lane: currently only start
Blood Channel in the medical community: As the main researcher, what information do you think of the announcement of this phase I clinical trial?
Professor Zhang Xi: CAR-T cell therapy is a "revolutionary" therapy in the field of tumor. It has also shown a good curative effect in patients with recurrence refractory acute B lymphocyte leukemia. However, the production cycle of traditional CAR-T treatment is long, and the FastCAR-T we use this time can compress the production time to 24 hours. In the first human test, strong cell proliferation and retention of T ability are displayed, and the toxic response is toxic. Controlled, it should be said that it has taken an important step forward.
Blood Channel of the medical community: We see that research is divided into 3 dosage groups. From the perspective of cell depletion and efficacy safety, there seems to be no obvious difference in the three dosage groups. I don't know what your evaluation?
Professor Zhang Xi: Fastcar-T itself does not have a long training process like our ordinary CAR-T. Its essence is actually the transformation from traditional in vitro expansion to expansion, so it enters the biological characteristics when entering the body's in vivo. And the mastery of the dose group is completely different from the traditional Car-T, so it is necessary to explore the appropriate dose of such cells. When we designed the initial dose, we were 1 magnitude higher than the traditional CAR-T (0.5 × 105), and then we carried out two upwards. Looking now, in fact, we have seen enough effect in the minimum dose group, and the overall CRS incidence rate is relatively high.
Therefore, if you want to conduct follow-up tests, we may need to lower at least one dose group, which may further reflect the true advantages of the FastCAR-T platform.
Blood Channel of the medical community: We see that the incidence of CRS in the study has reached more than 90%. Compared with the traditional CAR-T ratio, how do you evaluate its safety?
Professor Zhang Xi: I think the incidence of CRS is highly related to the characteristics of the cells itself. Basic studies have found that there will be more young T cells in the GC007F, the enlarged capacity in the body is stronger, and the peak time will be earlier.
In clinical practice, we see that some patients started to have a fever just after 1 day of infusion. On average, the symptoms of CRS reached the peak on average, and it did verify this.
As the number of clinical cases increases, we have more and more experience in CRS management. If necessary, we will choose to apply the monoclonal antibody of the beads, and even a small dose of glucocorticoids, which means that we must intervene and deal with it as soon as possible. No It will affect the proliferation and treatment effect of CAR-T. In the end, the CAR-T-related adverse events of us patients could be controlled well, and there was no death.
Blood Channel of the medical community: What information do you think of the ending of the patients with the traditional CAR-T?
Professor Zhang Xi: This study is a phase I study. The main endpoint indicators are dose restrictions, adverse events, and number of CAR-T cells. The number of cases included is relatively small. From the perspective of data, compared with the traditional CAR-T treatment process we have observed before, the FastCAR-T has better security. The pre -clinical research institute shows the advantages of cell dynamics, which needs to be further expanded to further expand the clinical study of samples.
references:
[1] zhang, c., he, j., liu, L.et Al.et Al.et Al.et Al.et Al.et Al.et AL.NOVEE's AntiGen Recept /doi.org/10.1038/s41408-022-00688-4
Expert Introduction
Professor Zhang Xi
Chief physician, professor, doctorate (post) mentor

Yangtze Scholars specially appointed professor
The Director of the National Hematology Center of Xinqiao Hospital of the Army Military Medical University, the whole army's blood disease center, the director of the Chongqing Hematology Medical Quality Control Center
Standing Committee of the Hematology Branch of the Chinese Medical Association
Executive Member
Executive Member of the China Anti -Cancer Association Hematology Special Committee
Chairman of the Chongqing Medical Society of Medicine Society
Chongqing chief medical expert
The first batch of Chongqing medical leaders
Chongqing Science and Technology Innovation Leadership Talents
The cutting -edge information you want
Please pay attention to the doctor station 注
1. Scan the QR code below
2. Click to download the app
3. Open the doctor station app and click on the upper right corner

4. Find the blood on the channel
Pay attention to

Download the doctor's station app and subscribe anytime, anywhere ~
The first release of this article: blood channel in the medical community

Author of this article: Yu Xiaosu
Review of this article: Zhang Xi
Editor in charge: Sweet
- END -
Realize the "electronic" analgesic drugs!Can you say goodbye to painkillers?

Everyone must have experienced the experience of somber desire. At this time, taki...
"Light your heart to see more hope" public welfare event will be officially launched on June 17, 2022

Wish is a good hope in my heart, expectations for beautiful things, and one of the...