CSCO Prostate Cancer Guide 2022 Update: Ora Pali combined with the Aibi Dragon treatment mechanism a
Author:Medical newspaper Time:2022.06.14

Research Background
Once prostate cancer progresses MCRPC, the prognosis is generally poor, and the survival is low. Patients who have metastasis in the distance, 80%of patients who have never metastasized in the past 5 years have never been transferred to 30%, and no progressive survival time is half of the patients who have not metastasized. Therefore, the first -line treatment of MCRPC is a critical stage in the process of disease treatment. Joint treatment has gradually become a hot spot for MCRPC's first -line therapy because it can bring better survival benefits to MCRPC patients. With the results of high -quality clinical research results, it will inevitably cause iteration and update of advanced PCA treatment solutions.
Phase III Clinical Study Propel confirmed that Orapali Union Abbit Dragon was the first treatment plan for the first -line video science in the first -line of MCRPC. It has a profound significance. The chapter of the "Diagnosis and Treatment of CSCO Prostate Cancer Guide (2022)" "Diagnosis and Treatment of Prostate Cancer", an Ora Pali combined with Abbiton as the treatment option for the MCRPC crowd. This article specially invites Professor Sheng Xinan of Peking University Cancer Hospital to interpret the treatment mechanism and Propel study of Ora Pali with Abbiton.
Ora Pali unite Abdo Dragon
Joint treatment mechanism
PARPI (PARPI) represented by Ora Pali has previously proven MCRPC patients who can carry homologous restructuring (HRR) gene mutations through "synthesis death". PARP1 and PARP2 are part of the DNA damage reaction mechanism, which is essential for maintaining DNA integrity. PARPI can capture PARP1 and PARP2 enzymes at the damaged DNA. The complex formed causes DNA to copy stagnation and DNA breaks, which produces more cytotoxicity than the unwanted single chain breaks caused by the innocent of the pure PARP. effect.
The occurrence ofrogens and PCA are closely developing, and the drug (such as ARAT Dragon) of the subject (ARAT) of therogens receptor axis (such as Abbit Dragon) brings obvious survival benefits to patients with advanced PCA. PARP can not only interact with the transcription factors ERG, but also regulate the combination of AR and chromatin to inhibit PARP PCA cells sensitive to DNA damage androgen consumption. In addition, the DDR signaling pathway (including HRR genes) and AR signaling pathways in PCA are crossing. AR signal inhibitory or lack of AR can lead to a reduction in HRR expression.
It is speculated that the decline in drug -induced AR function has reduced the expression of HRR genes (such as BRCA1, RAD51AP1, RMI2, etc.). Therefore, drugs targeting AR and PARP drugs such as Ora Pali can produce synergistic lethality in PCA.
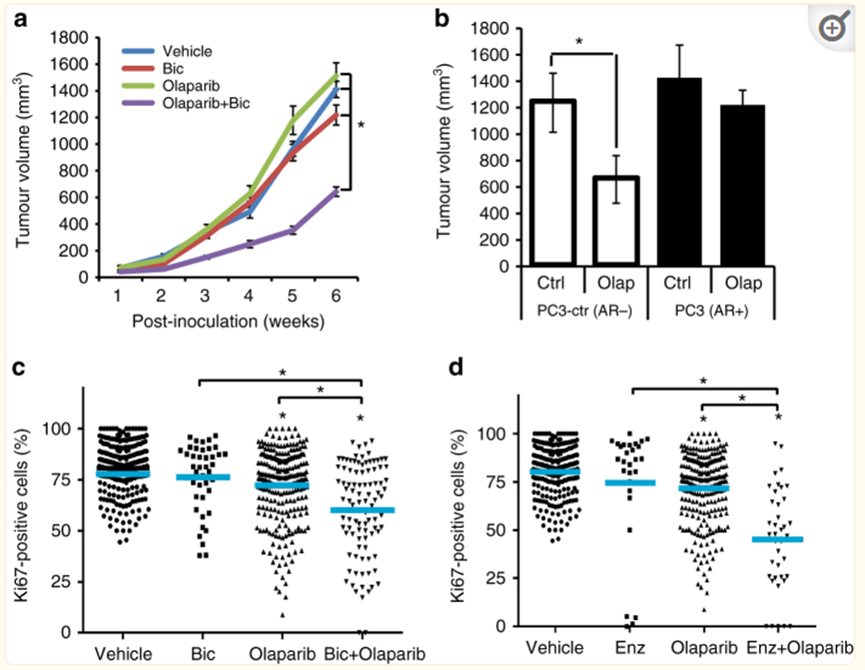
DUAL Inhibition of ARAND PARP1/2 Function Represses PCA Growth.
The dual inhibitory effects of AR signal and PARP1/2 inhibit PCA growth
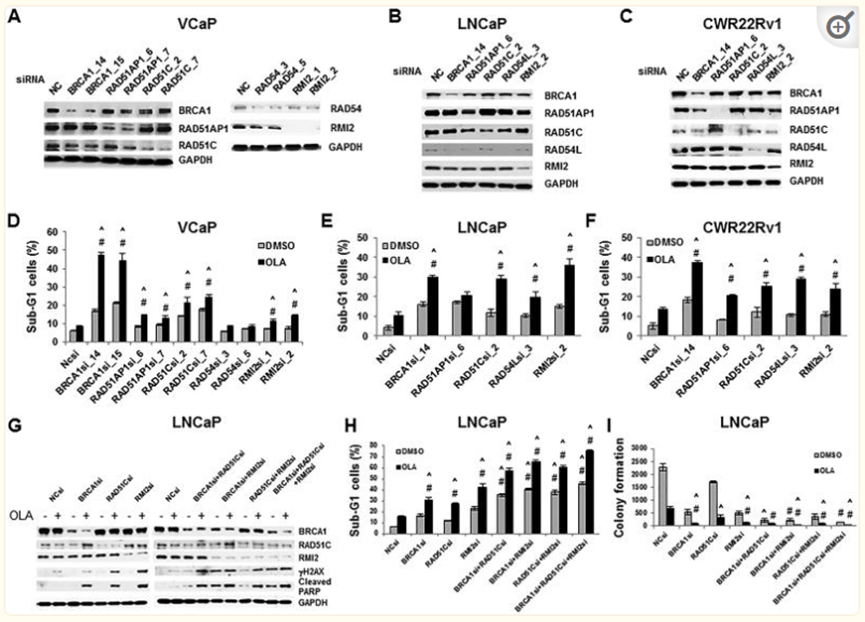
Hrrr gey SilenCingsynergizes with Ola to Increase Cytotoxicity to PCA Cells
HRR gene of silence and OLA coordinated increased cytotoxicity to PCA cells
In short, Ora Pali weakens the transcriptional activity of AR channels, and ARAT (Abbit Dragon) can induce HRR defects and reduce DDR gene transcription, which has a certain effect on Parpi. Therefore, Olapali and Abbit Dragon are united to play a synergistic anti -tumor role of 1+1 & 2, and it has nothing to do with whether HRR's HRR of PCA patients has nothing to do.
Study 08 in Phase II Clinical Studies shows that Olapali combined with Abbit dragon treatment brings significant clinical benefits to MCRPC patients (regardless of HRR mutation status), and the overall safety is good. Subsequently, the joint therapy entered the phase III PROPEL study to confirm it, and verbally reported the research results on the 2022 ASCO-GU. The full text was published in the NEJM subscription "Nejm Evidence".
Propel research interpretation
Research purposes
It is confirmed to prove the efficacy and safety of Olapali unite in the first crowd of MCRPC.
Research design
Propel is a global multi -center, random, double -blind phase III clinical study. 796 patients with first -line MCRPC were included. The patients who were admitted did not consider the state of HRR mutation. )therapy group. The baseline of the two groups of patients was balanced, especially the baseline of the HRR mutation ratio of the two groups of patients. The main endpoint of the study is the images of the images of no progress (RPFS) evaluated by the researcher, and at the same time explore multiple secondary end points including the total survival period (OS).
Research result
Main end point RPFS:
Compared with the treatment of Aubili combined with Aibi Dragon and Abbit Dragon, the median RPFS evaluated by the researcher was extended for 8.2 months (24.8VS 16.6 months, HR 0.66, 95%CI 0.54-0.81, P <0.0001) , Reduced the risk of progress or death by 34%, and no matter what the patient's HRR status is; BICR evaluation shows that combined therapy comparative drugs can extend the median RPFS for 11.2 months (27.6 vs 16.4 months, HR0.61, 95% CI 0.49-0.74, P <0.0001), reduced the risk of 39%of imaging progress or death; the Asian group analysis showed that the RPFS of all Asian groups improved, including the hro-enriched tumor tissue and circulatory tumor DNA detected Prompt patients (HR 0.50, 95%CI0.34–0.73) and patients who have not detected HRR mutations (HR 0.76, 95%CI0.60–0.97). Key secondary end OS:
Although the data is immature (228 incidents have occurred and the data maturity is 28.6%), the trend of Ola Pali combined with the Abbit dragon treatment group has been observed (HR 0.86, 95%CI 0.66-1.12 , P = 0.29).
Other secondary end points:
A.TFST and PFS2 Ala Pali combined with Abbit Dragon, TFST (HR 0.74, 95%CI 0.61-0.90, P = 0.004), PFS2 (HR 0.69, 95%CI 0.51-0.94, P = 0.0184 To.
b. Research safety analysis combined treatment reflects the predictable security characteristics, and most patients can treat the progress of the disease. The analysis of adverse events shows that the combined treatment is consistent with the characteristics of the two known single drugs. The most commonly reported adverse events that are most commonly reported are anemia (15.1% vs 3.3%), and no MDS or AML is reported. Essence
c. The combined treatment group after the treatment of prostate cancer was compared with the Aibi Dragon Single Pharmaceutical Treatment Group. The quality of life of the two groups was different.
Look forward to
MCRPC is the end of the PCA. Although the new endocrine drugs represented by Abbiton have been widely used in clinical use, the current clinical study shows that the median OS patients in MCRPC are still less than 3 years. In the year, there was a great failure to meet medical needs. The PROPEL study results provides evidence -based medical evidence for Olapali and Abbiton's use of the front line of MCRPC. Joint therapy can bring significant clinical benefits to patients with MCRPC. With the maturity of OS data, it is expected to further reveal the long -term survival benefits of Olapali combined with Aibi Dragon treatment in clinical practice.
Expert Introduction
Professor Sheng Xinan

Chief physician, associate professor, doctoral supervisor
Deputy Director of the Department of Internal Medicine of Urology and Cancer Hospital of Peking University
Director of the China Clinical Oncology Society (CSCO)
CSCO Youth Expert Committee Standing Committee Member
Secretary of the CSCO kidney cancer expert committee
Standing Committee Member of the Expert Committee of CSCO Urinary Cancer Cancer
Executive Director of the Youth Council of the China Anti -Cancer Association
Member of the Kideline Cancer Group of the China Anti -Cancer Association Urology Cancer Special Committee
Deputy Leader of the Cancer Team of the Youth Committee of the Chinese Medical Association of the Chinese Medical Association
Chairman of the Youth Committee of the Beijing Anti -Cancer Association's Urology Cancer Specialization Committee
Deputy Leader of the Urology Group of the Rare Disease Branch of the Beijing Medical Association
References: (slide and view)
not
1. SCHIEWER MJ, ET Al Cancer Discov 2012; 2: 1134–1149;
2. Goodwin jf, et al.cancer discov 2013; 3: 1254-71;
3. Asim M, et al.nat Commun 2017; 8: 37
4. Fred Saad et al., ASCO-GU 2022, CA, Abstract 11;
5. Hussain m, et al.New English J Med. 2020; 383: 2345-2357.
6. Loriot y, et al.annn oncol.2013; 24 (7): 1807-1812.
7. badrising s, et al.cancer.2014; 120 (7) 968-75
8. SWAMI U, et al.cancers, 2021.13 (19) 4951.
ES. Parp inhibitors in metastatic avcer: evidence to date. CANCER Manag Res.2020 SEP 7; 12: 8105-8114. Doi: 10.2147/cmar.s227033.
12. Li L, Karanika S, Yang G, et al.androgen Receptor Inchibitor-indenced "Brcness" and Parp Inhibition are synthetical -Ressantial can;
Capture: Liu Zibo
Edit: Yin Yan
- END -
Henan two places reminded: these people please report immediately
Emergency reminderIn order to strictly implement the measures of external prevention input and internal prevention and rebound, effectively control and reduce the risk of transmission of new crown p
National Health and Health Commission: In the past ten years, the incidence of AIDS disease and tuberculosis report in my country decreased significantly

Cover news reporter Shao MengOn June 17, the National Health and Health Commission...