Answers of the safety effectiveness of the new crown virus vaccine (1)
Author:Healthy China Time:2022.07.23

On July 23, the State Council's joint prevention and control mechanism held a press conference. The responsible comrades and relevant experts of the National Health and Health Commission attended the meeting to introduce the relevant situation of the safety effectiveness of the new coronary virus vaccine. Please pay attention to the hot Q & A of this conference!

Quickly curb the spread of the local epidemic, strictly implement various measures for foreign defense input
△ Mi Feng, spokesman for the National Health Commission and Deputy Director of the Propaganda Department
According to the World Health Organization report, last week's newly -added diagnosis cases in the Western Pacific region of China increased by 37%, which is the fastest growing area in the world. Omikon BA.5 Asian branches have spread to more than 100 countries and regions around the world, leading to an increase in the number of infections, hospitalization, and severe illnesses, and China's foreign defense input pressure has continued to increase.
Recently, the local epidemic has shown a multi -point and frequent trend, and the outflows of the epidemic in some areas have not been completely blocked, and the situation of preventing and control is severe and complicated.
It is necessary to adhere to the overall strategy of "external prevention input, internal defense rebound" and the general policy of "dynamic clearing zero". The areas where the epidemic occurs in the area due to local treatment, concentrated resources and strength to quickly extinguish, quickly curb the spread of the local epidemic spread Spread; border areas and inbound cities must strictly implement various measures in the air defense input, strictly closed loop management, and resolutely avoid the prevention and breakfast of the prevention and breakthroughs; The system is operating efficiently. We must continue to do a good job of vaccination of the new coronary virus.
The previous conference mentioned that the vaccination rate of new crown vaccines in the elderly group in my country was low. Does this situation improve?
After being approved in my country's vaccine listing, there is actually a complete set of regulatory processes to ensure the quality of the vaccine.
First of all, before the factory is issued, enterprises must be inspected in accordance with the national approval standards. The inspection items include physical indicators, chemical indicators, identification indicators, validity indicators, and safety indicators. The following important steps, the national legal inspection agencies need to be approved to issue the vaccine. Corresponding issuance is the unified requirement of biological products internationally. The same is true of my country, and the legal inspection agencies are required to inspect and review each batch of vaccines that are about to be listed. The inspection report is issued after the inspection is qualified to meet the standard, allowing release. In other words, before the vaccine was listed, it was necessary to be listed after the inspection of the enterprise and the test of the national legal institution.
In addition, after the new crown vaccine was launched, the production increased sharply, which was unprecedented. In order to ensure the quality of the vaccine in production, the national legal institutions have regulated the provinces of the provinces, expanded the ability to replenish the ability of approval, and improve the ability of batch issuance. At the same time, the pharmaceutical supervision institution also dispatched a inspector in the factory to the vaccine manufacturer to check the entire process of vaccine production to ensure that the production process meets the requirements of national regulations.
After the vaccine finished product is leaving the factory, there is a series of measures to ensure the quality of the vaccine during use. For example, the production line expansion work process and technical guidelines have been introduced, and an independent operation of the new crown vaccine information traceability regulatory system is established to ensure that each vaccine source can be chased and the direction can be checked. Through the above series of measures, the safety and reliability of the new crown vaccine listed on the market is ensured.
Recently, there are rumors on the Internet. Some people have diabetes and leukemia after vaccination with new crown vaccines. Is this situation true? What should we look at this question?
It can be clearly told everyone that vaccinating new crown vaccines will not cause the occurrence of leukemia and diabetes, nor will it affect the genetic development of the human body, cause tumor metastasis and spread, and cause antibody dependence to enhance (ADE). Unsifferently wrong words on the Internet.
Let me analyze it from three aspects.
First, various substances in vaccines are safe to the human body and will not directly cause disease. Activated vaccine mainly contains microphone -level virus antigen or recombinant proteins, as well as other ingredients such as aluminum hydroxide agents and auxiliary materials. Their content in vaccines meets relevant regulations and cannot lead to disease.
Secondly, the largest number of new crown viruses in my country currently has a sufficient security guarantee, and has been recognized by international organizations. It is basically the same as that of the production of hepatitis A vaccine, rabies vaccine, and spinal ashgiitis vaccine, which have been listed at home and abroad for decades, and have not seen reports that have been associated with leukemia or diabetes. After the new crown epidemic, a number of new crown virus vaccines developed in my country have been included in the World Health Organization's emergency use list. It has been used in more than 100 countries, achieving the expected results, and security meets international standards.
Third, clinical monitoring and statistical data show that during the four years before and after the new crown epidemic, the number of diabetes and leukemia is basically the same as the number of hospitalization and the number of hospitalizations, which indicates that the vaccination of the new crown vaccine will not cause leukemia and diabetes.
Based on the above analysis, we can be sure that the vaccination of the new crown vaccination will not cause leukemia and diabetes.
Elderly elderly people over 80, such as disability and living alone, rarely go out and rarely contact the outside world. Is it necessary to vaccinate?
These elderly people should actively vaccinate the new crown virus vaccine. Because with the significant increase of vaccine vaccination coverage and the variation of the virus, asymptomatic infection is increasing. my country is a country with a strong family. If the family is an asymptomatic infected or some of our friends is an asymptomatic infection, we will return to our house or our relatives and friends to visit the old man, and we will bring the risk of infection to them Before you. Once these elderly people are infected, and they have not vaccinated vaccines and have not carried out vaccines to strengthen needle vaccination, the risk of severe and death will increase significantly.
Therefore, the elderly must be accelerated. Only when the vaccination rate of the elderly, including the vaccination rate of the vaccine enhancement needle, can increase the risk of severe illness and death caused by the new crown virus to the elderly themselves. From another perspective, you can also take the initiative to prevent and control the country's epidemic.
Is the current vaccine still valid for the Omikon variants? What is the progress of the research and development of the Vaccine of Omikon's mutations in my country?
Compared with the previous mutant strains, the degree of mutation of Omircong is indeed a relatively large degree of mutation, and the immune escape ability is relatively strong. It has a certain impact on the existing vaccines and the antibodies of the human body induced by previous infections. But immune protection not only depends on neutralized antibodies, but also related to cellular immunity and immune memory. Therefore, the results of multiple studies show that the effects of existing vaccines at home and abroad still maintain a high level. my country's vaccine still has a good protective effect on the severe disease and death caused by the mutant strains of Omikon, and strengthening immunity can further reduce the risk of hospitalization, severe and death.
Regarding the vaccine research and development of mutant strains, the State Council joint defense and control mechanism science and technology group vaccine research and development special classes were listed on December 26, 2021, the World Health Organization listed Omikon mutant strains as a major mutant strain, that is, organization experts, that is, organization experts Research and judge, start the deployment of research and development.
At present, a number of technical routes have developed the development of unit prices and multi -price Omikon variants. The fast -moving unit price of Omikon variants has been approved by clinical trials and is conducting clinical trials in Zhejiang, Hunan, Hong Kong and other places. The rapid progress of the tetraonal restructuring protein vaccine has been approved by the Phase III clinical trial of the UAE, and related research has been launched. Many other unit -prices, multi -price containing Omiro Rong mutant live vaccines, reorganized protein vaccines, adenovirus vector vaccines, and MRNA vaccines are also conducting pre -clinical research. Essence In addition, the special class also deployed the research on the new -spectrum vaccine, and the rapid progress of vaccine has been approved by clinical trials and launched related research.
In general, the research and development of the Omicor Rong mutant plant vaccine in China is steadily promoting. As long as there is a need, in accordance with the requirements of relevant regulations, the emergency application procedures can be quickly launched to provide vaccination.
Text finishing: Wang Ning
Video editing: Wang Ji Ke Pu Qinying
Edit: Chen Xiuchao
- END -
Before the benchmarking heart, improve the party nature!Shangma Street held 2022 party activists training classes in 2022

From June 20th to 24th, Shangma Street held a training course for activists joinin...
Chengdu today adds local "2+2" at 0-14 o'clock
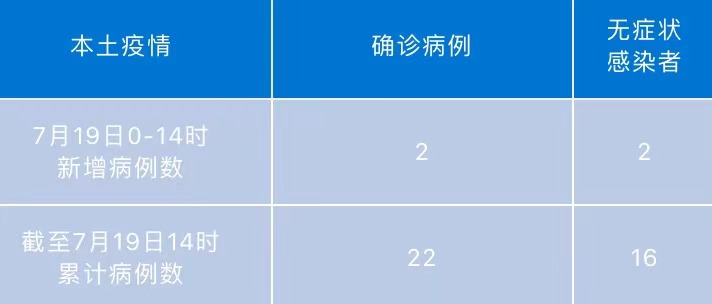
At 0-14 on July 19, there were 2 new local diagnosis cases in Chengdu, and 2 cases...