Conditions for oral new crown drugs independently developed in my country are approved
Author:Jinan Daily Time:2022.07.26
On July 25, the State Drug Administration conducted emergency review and approval in accordance with the relevant provisions of the Drug Management Law in accordance with the relevant provisions of the Drug Management Law.Symptom registration application.
This product is the treatment of oral small molecular virus pneumonia for oral small molecular virus pneumonia in my country.On July 20, 2021, the State Drug Administration has attached conditions to approve this product and other reverse-translitease inhibitors to treat adult HIV-1 infection patients with high virus load.This is an attachment to approve new indications for the treatment of adult patients with COVID-19) for the treatment of ordinary new type of coronary virus pneumonia.Patients should be used strictly under the guidance of the doctor.
The State Drug Administration requires listing permit holders to continue to carry out relevant research work, complete the requirements for qualified conditions within a time limit, and submit subsequent research results in a timely manner.(Source: official website of the State Drug Administration)
Edit: Zhang Yu
- END -
The fuel cost of domestic routes has risen 5 consecutive, which is the highest collection standard for 20 years | See the cover every day
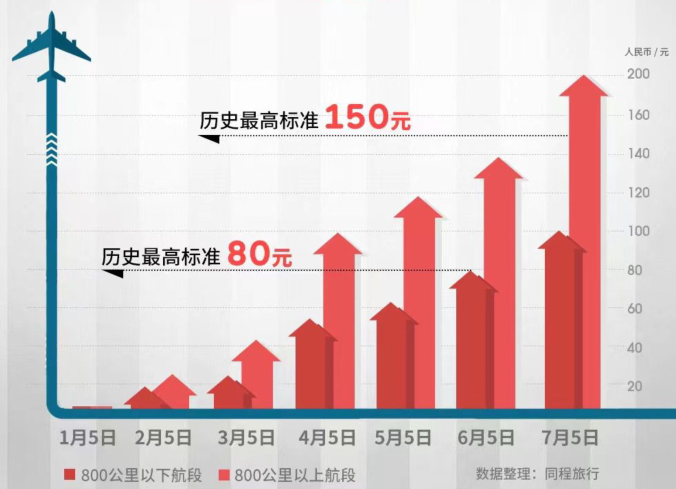
Cover reporter Wu YujiaOn June 30, Tongcheng Travel stated that he received a noti...
Announcement on the announcement of typical cases of typical cases of violations of high -level civil building fire safety management in the Gansu Provincial Fire Rescue Corps

The Provisions on Fire Safety Management of high -rise civil buildings has been im...