T-DXD has a significant clinical benefit and extensive layout, providing a better solution for patients with advanced gastric cancer in HER2
Author:Cancer Channel of the Medical Time:2022.07.29
*For medical professionals for reading reference
Based on mechanisms and based on evidence, T-DXD has promoted in-depth research in the field of advanced gastric cancer in HER2. From the forward movement of the treatment front to the exploration of the joint plan, the multi -dimensional broaden -beneficiaries will be widened, with a view to provide treatment options for more patients.
HER2 is one of the important targets of gastric cancer. The proportion of expression or genetic amplification in gastric cancer is about 12%-20%[1]. The combined treatment of Tolkuzumab establishes the first-line treatment position of HER2-positive gastric cancer. Keynote-811 research [2] further confirms the importance of anti-HER2 treatment, but the choice of anti-HER2 therapy for such patients is still limited, and urgent needs Further clinical benefits. The emergence of new antibody drugs (ADC) provides new treatment options for patients with advanced gastric cancer in HER2. At present, ADCs have approved indications at home and abroad and are recommended by guidelines.
At present, ADCs with HER2 as the target in the gastric cancer field mainly include T-DXD, RC48 and ARX788. Among them, T-DXD (DS-8201, ENHERTU) is the first ADC drug in the world to treat HER2-positive gastric cancer. It has obtained the approval of Japan (MHLW) and the United States (FDA) for advanced HER2-positive gastric cancer patients. Line treatment. The breakthrough effect and good safety of T-DXD in ADC drugs are inseparable from its unique drug design and action mechanism.
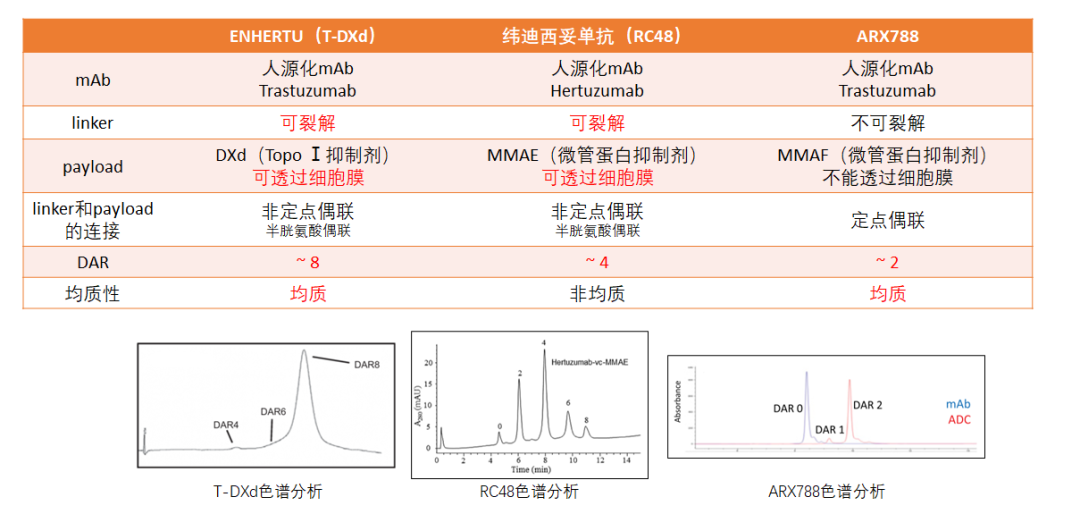
Figure 1. Comparison of anti-HER2-ADC structure in the field of gastric cancer [3-7]
(Note: See chromatography analysis diagram of the average quality of each drug)
Unique! T-DXD has emerged in the ADC track with exquisite design and unique mechanism
The ADC is formed by a monoclonal antibody and a strong killing cytotoxic drugs. After connecting the covalent of the sub -covalent, when the antibody and the surface antigen of the target cells are combined, the ADC is swallowed into the tumor cells, and then occurs in the lymphatic body. Discretion, release high -active drugs, destroy DNA or prevent tumor cell division, and play a role of tumor killing [8].
The ADC combines the dual advantages of targeted, selective antibodies and high antitumor active cytotoxic drugs. It can efficiently and accurately kill tumors, which greatly increases the risk of benefit of anti -tumor treatment. As the level of R & D has continued to improve, the current ADC has developed to the third generation, the most striking drugs are T-DXD.
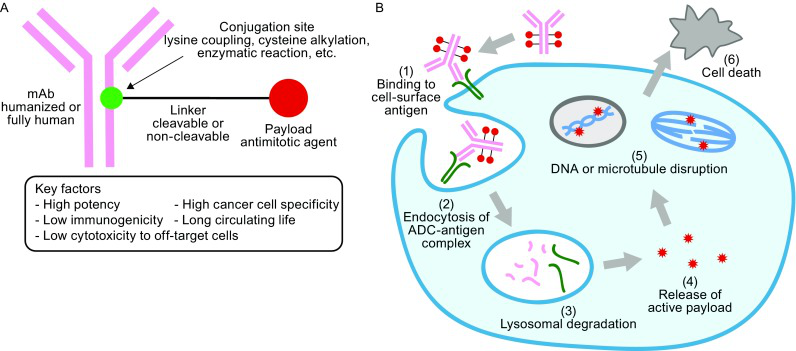
Figure 2. Dragons of the structure and action mechanism of ADC drugs [8]
The T-DXD is converted by the new DNA topological enzyme Ⅰ inhibitors that target the humanized monoclonal monoclonal antibody Telkomo Mipida that is targeted at HER2. T-DXD adopts cutting-edge design concepts, with multiple differentiated highlights:
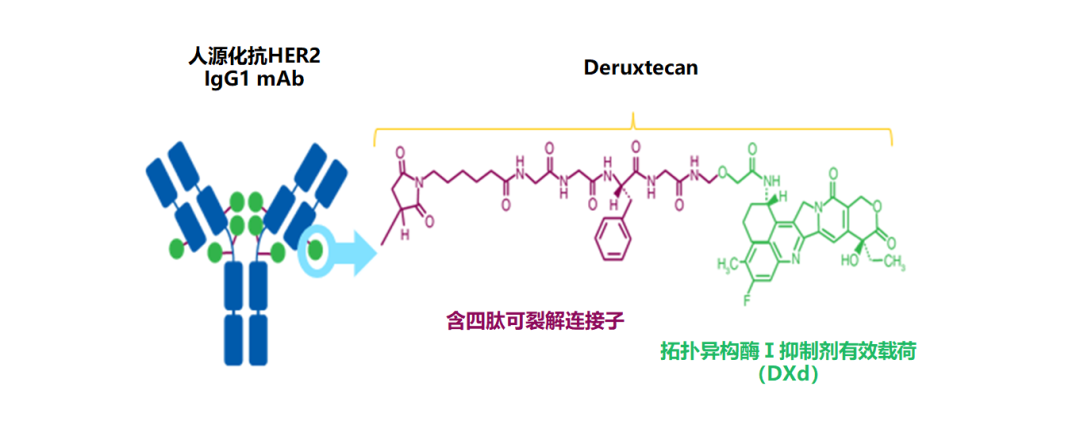
Figure 3. T-DXD compound structure diagram [6]
Optimization of cracking connection sub -structure, to maximize the effect while ensuring system security
T-DXD uses a cracking connector based on the GGFG tetrapeptide sequence. After being swallowed by the target cells, the lympicine protease specific identification and cutting are cut and cut by the highly expressed mesurase in the tumor cells to achieve the efficient release of the carrier. Choose cysteine Optorizing method, controlling the capacity of single antibody while ensuring the meanness of the drug, so that the T-DXD drug antibody ratio (DAR) ratio (DAR) reaches 8, surpassing existing ADC drugs, greatly enhanced the effectiveness of anti-tumor; The DAR distribution of T-DXD is uniform, which helps to transport more drugs to target cells, thereby exerting a stronger tumor killing effect [9-11].
In theory, the higher the drug concentration, the stronger the anti -tumor effect. It is inferred that the higher the DAR, the better. However, excessive DAR may cause problems such as poor ADC stability and high decisive toxicity. Therefore, the previous concept believes that the stability and security of ADC with 2-4 are more advantageous [12]. However, the DAR of ARX788 is only 2, but it has obvious eye toxicity [13]. The synthesis of T-DXD uses optimized connecting sub-technology and does not have the above problems. And compared with the traditional aminotype (PAB) connection (such as SYD985 and RC48), the T-DXD tetrapeptide connecting sub-hydration is lower. Stable while ensuring sufficient effective intracellular drug concentration [14]. In addition, although the connected sub -cracked, it has high stability in the blood circulation. The drop rate of DXD is extremely low, which can greatly reduce the systemic off -target effect to a large extent. Studies have shown that the release rate of DXD in plasma in 21 days is only 1.2%-3.9%. Compared with it, the release rate of T-DM1 at 4 days is as high as 18.4%[9]. Not only that, the semi -period of the system of free DXD is only 1.37 hours [15], which can discharge the body faster, and quickly reduce the concentration of blood medicine, which helps further reduce non -target toxicity.
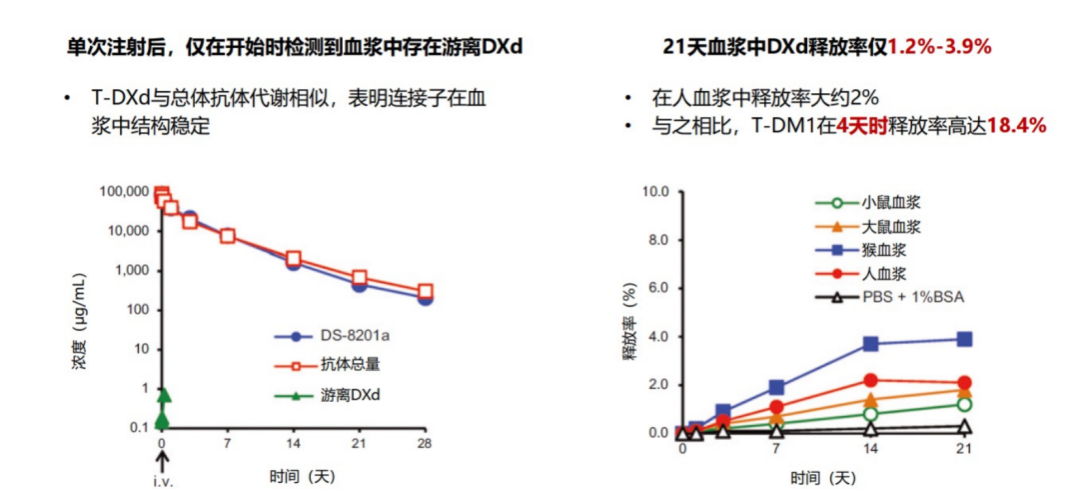
Figure 4. The connection of T-DXD is stable in the plasma, and the drop rate of drugs is extremely low [9]
The high -active medicine of the new mechanism can avoid drug resistance and strong anti -tumor activity
Drugs are the core composition of ADC, which can kill tumor cells directly or through bystanders. T-DXD's carrier is a new type of topological enzyme Ⅰ inhibitors DXD, which mainly has the following characteristics: on the one hand, DXD is different from the commonly used chemotherapy drugs, which can avoid cross-resistant drugs with previous treatment; second, DXD is strong Anti-tumor activity is 10 times that of Topotomy is also the topology heterase I inhibitor SN38 (Ilidekang's active metabolites), while the activity of SN38 is 100-1000 times that of Ilidekang; more importantly, DXD, DXD It also has the characteristics of membrane permeability, which is one of the necessary conditions to meet T-DXD to play the effect of the onlookers [6,7]. A strong onlooker effect, enhance tumor killing effect and effectively overcome the heterogeneity of tumor
Based on the characteristics of the connected sub-cracked and the permeability of the drug, the T-DXD has an onlooker effect. Based on the high DAR and high-active drugs, the onlookers of T-DXD have greatly strengthened. It is worth mentioning that its onlooker effect only killed the neighbor's tumor cells and had no effect on the distant cells, that is, while ensuring the efficacy, ensuring system security [10].
As we all know, HER2 expression is heterogeneous. Anti-HER2 treatment will screen tumor cells, killing sensitive cells (HER2 high expression, IHC3+and IHC2+/ISH+), and HER2 low expression (IHC1+and IHC2+/ISH-) Cells obtain a proliferation advantage, leading to drug resistance. However, the T-DXD has a powerful onlooker effect. While killing HER2 highly expressing tumor cells, it can also perform effects on HER2 low-expression tumor cells [9], effectively overcome the heterogeneity of tumor and delay drug resistance.
Figure 5. The anti-tumor activity of T-DXD depends on the medicine [9]
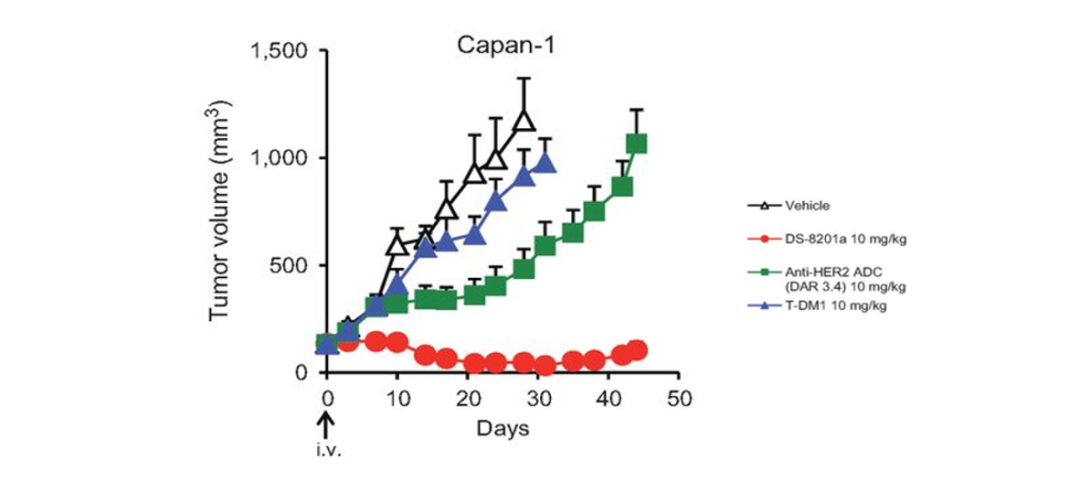
In summary, the T-DXD uses optimized connectors, which can maintain stable and reduce desertotoxicity in the blood, but also can be effectively released by enzyme protease specificity. In addition, cracking connections, diaphragm and high activity, and DAR of up to 8 make the T-DXD have a strong onlooker effect, killing tumor cells while killing tumor cells. In short, the drug characteristics of T-DXD are the basis for its breakthrough clinical benefits. At present, T-DXD single medicine or combined therapy has carried out a number of clinical research in HER2 positive gastric cancer.
Dajian! T-DXD extensively layout HER2 positive advanced gastric cancer treatment
Figure 6. Research and summary of T-DXD in HER2-positive gastric cancer
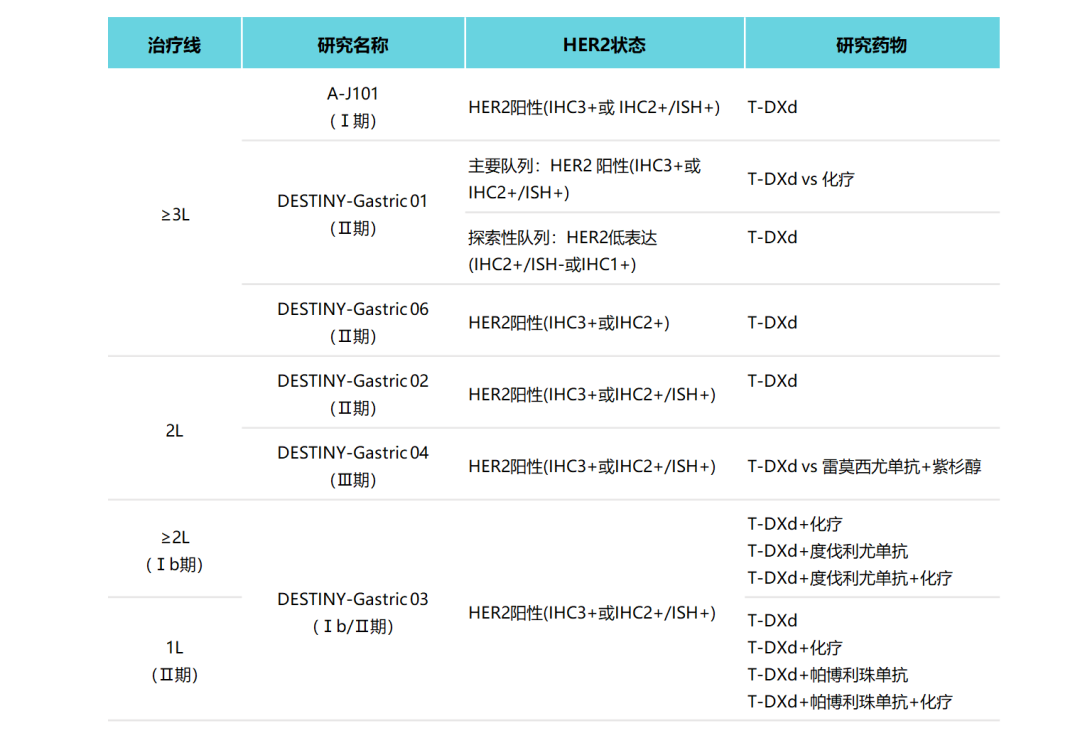
01
Broadly! T-DXD has a significant effect among HER2 positive patients
1. A-J101 Study-Preliminary Exploration of T-DXD Drugs in physical tumors
A-J101 studies the safety and tolerance of T-DXD in patients with advanced physical tumors. PART1 is an increasing study. PART2 is a dose expansion study, including multiple queues. Among them, 2B queues are included in HER2 positive, and the gastric or gastroesophageal adenocarcinoma (hereinafter referred to as gastric cancer) at the junction of gastric or gastric pipes (G/GEJ) In patients, RP2D is 6.4mg/kg.
Figure 7. A-J101 Research Design [16]
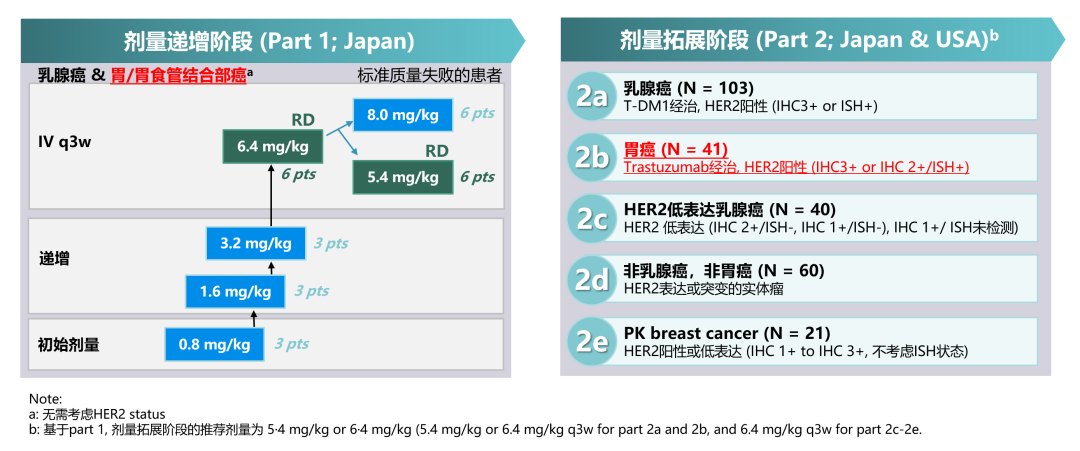
There were 44 patients in this study, and 27%of patients had previously received at least 5 -line system treatment. The results show that the T-DXD single-drug treatment can be tumor, and the objective relief rate (ORR) is as high as 43.2%, and the mid-level relieving duration (DOR, 7.0 months), no progressive survival period (PFS), and medium in medium level, medium and medium, medium and medium The total survival period (OS) is 5.6 months and 12.8 months, respectively. Level ≥ 3 Teae is 64%, and drug -related deaths caused by AE [16].
Overall, the efficacy data of T-DXD is encouraged and safe. The A-J101 study initially confirmed the good anti-tumor activity of T-DXD in the field of advanced gastric cancer, and then opened a highly anticipated Destiny series research.
2. Destiny-Gastrid 01 Research-The first random control research to confirm T-DXD clinical benefits in the field of gastric cancer
Destiny-Gastrid 01 Studies compared the efficacy and safety of the chemotherapy (paclitaxel or Ilidekang) chosen by T-DXD and doctors in patients with advanced gastric cancer in Japan and South Korea. The research results were synchronized in the 2020 ASCO conference and "Nejm). A total of 187 patients were included in the study, and the experimental team had 20%of patients who had experienced four lines and above. The preliminary analysis results show that the efficacy and safety of T-DXD are generally consistent with the results observed in other clinical studies in T-DXD in Phase I and previous reports [17]. The final OS analysis of the update in 2022 shows that in terms of efficacy, the median OS in the T-DXD group has 12.5 months, which is significantly better than the chemotherapy group (8.4 months); More than double (14.3%); DCR is 85.7%and 62.5%, respectively, and the median PFS is 5.6 months and 3.5 months, respectively. In terms of security, new bad events have not been observed, and the overall safety is good. 16 patients with T-DXD-related interstitial lung disease (ILD), most of them are level 1 or 2, and no level 5 ILD occurred [18]. Figure 8. Destiny-Gastric 01 Research Design [17]
Her2 -positive gastric cancer patients who have progressed at least two types of treatment often have poor ending. The Study of Phase I-J101 preliminary shows that T-DXD has a significant advantage in the tumor relief rate and overall survival period when treating such patients. The stage II Destiny-Gastrid 01 study confirmed the excellent effect of T-DXD in the patient group. T-DXD is the first anti-HER2 targeted drug that has been confirmed by random control research compared with chemotherapy that can significantly extend the time of survival, and starts a new situation of ADC drug therapy HER2 positive advanced gastric cancer. Based on Destiny-Gastric 01's main queue, in May 2020 T-DXD was awarded the Breakthrough therapy (BTD) by the US FDA, and was approved by the FDA in January 2021 to treat the Tuskuzumab solution HER2 positive advanced gastric cancer patients.
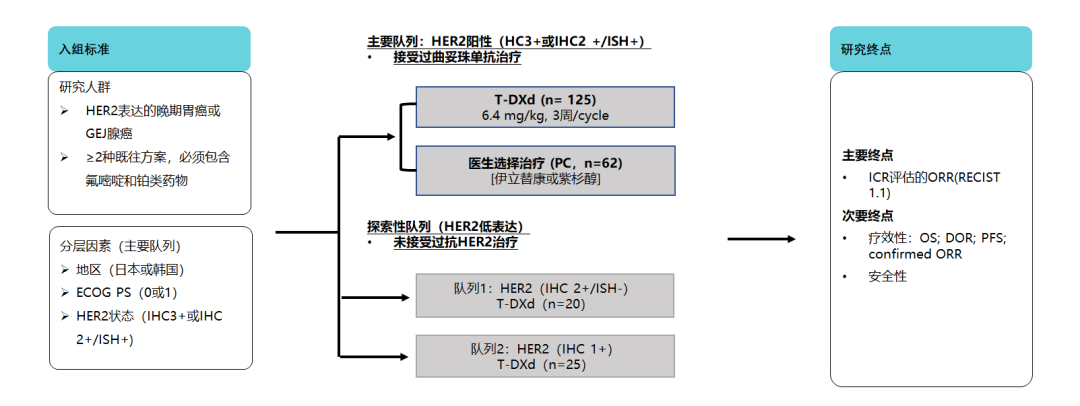
并且,2020年ESMO年会中发布的探索性队列研究结果显示,在IHC 2+/ISH-队列中,ORR达36.8%,中位经确认的DoR达7.6个月;在IHC 1+队列中, ORR reaches 19%, and the medium is confirmed by 12.5 months [19]. The results of this study show that T-DXD can help HER2 lowly express patients to achieve survival benefits, explore the efficacy and safety of T-DXD in different HER2 expressing gastric cancer patients, and further prove the structural advantage of T-DXD.
Figure 9. T-DXD is used in HER2 low expression, realizing long-term benefits [19]
Third, Destiny-Gastric 06 Research-The efficacy of T-DXD in the third line and later Chinese patients will be verified
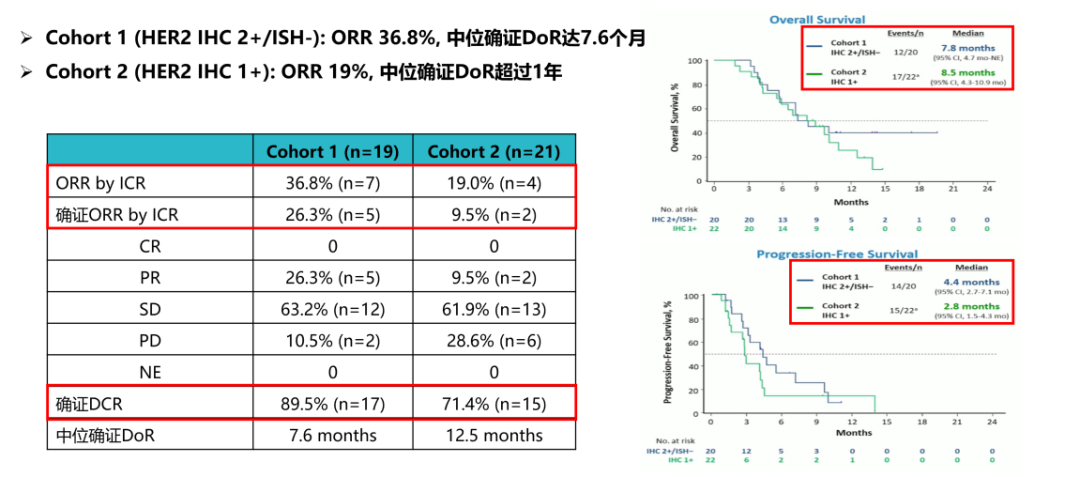
Destiny-Gastrid 06 Study among Chinese HER2 positive and previously treated patients with at least 2 systematic treatment, evaluating the efficacy and safety of T-DXD 3 and above treatment. It is Destiny-Gastrid 01 research in the bridge test in China [ 20]. The study is running smoothly, and the announcement of the subsequent results will continue to consolidate the position of T-DXD in the field of advanced gastric cancer in HER2-positive patients.
Figure 10. Destiny-Gastric 06 Research Design [20]
Fourth, Destiny-Gastric 02 Research-Re-verification in European and American populations
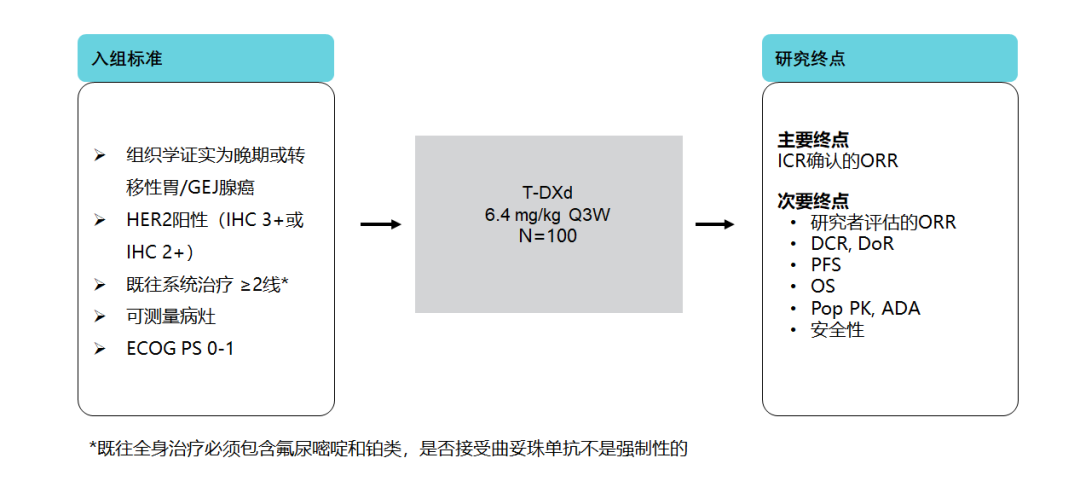
The study evaluated the efficacy and safety of T-DXD second-line single-drug treatment HER2 positive insectable or metastatic gastric cancer in the Western population. The study was included in 79 patients. In terms of efficacy, ORR reaches 38%, DCR is 81%, and the median DOR length is 8.1 months. The incidence of pneumonia is 7.6%, most of which are level 1 or level 2 [21].
Figure 11. Destiny-Gastric 02 Research Design [21]
5. Destiny-Gastric 04 Research-T-DXD second-line single-medicine head comparison standard scheme
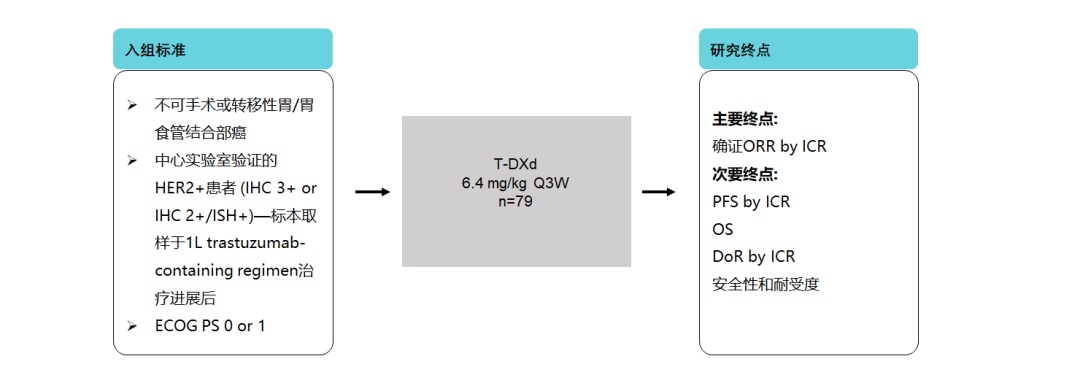
In 2022, the "CSCO Gastric Cancer Diagnosis and Treatment Guidelines" uses Remo Missu Mipidic combined with paclitaxel as a second-tier 1A scheme for patients with advanced gastric cancer patients in HER2. The efficacy and safety of T-DXD single drugs and standard schemes [22].
Figure 12. Destiny-Gastric 04 Research Design [22]
02
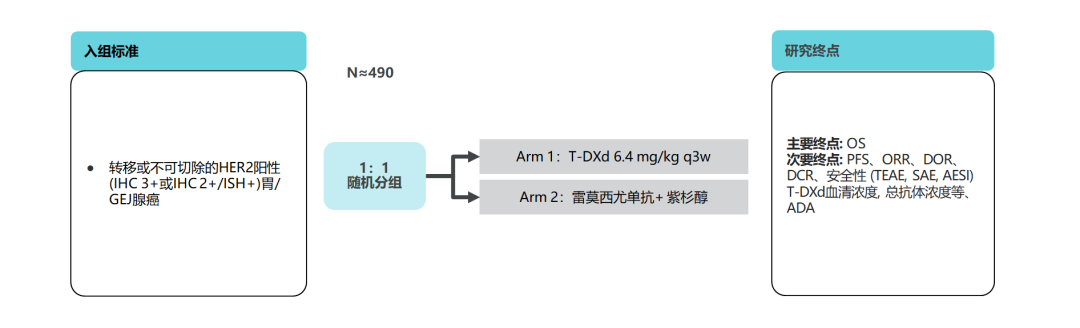
Chase the victory! Explore a new direction with T-DXD as the core, in order to benefit more patients
Destiny-Gastrid 03 Research-T-DXD combined treatment mode is underway
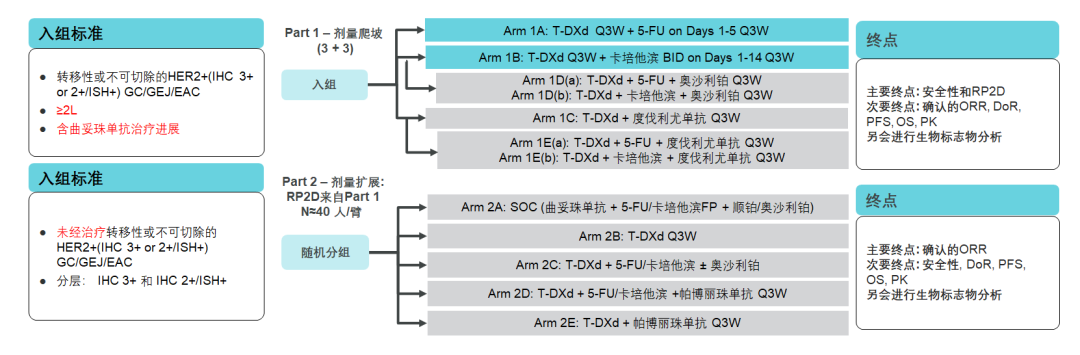
The above studies are concentrated in the efficacy and safety of T-DXD single drugs in patients with back-line patients. Destiny-Gastrid 03 Studies will reveal the efficacy and safety of the joint scheme with T-DXD as the core. Destiny-Gastrid 03 Research aims to evaluate the safety and preliminary antitumor activity of T-DXD single drugs or combined treatment, and include patients with advanced local or metastatic gastric cancer (IHC 3+ or 2+/ISH+) in North America, Europe and Asia. Essence PART1 is an increasing study, which is included in HER2 -positive gastric cancer patients who have progressed after the treatment of the Twolfuzumab; PART2 is a dose expansion study, which is included in patients with HER2 -positive gastric cancer who have not been treated and confirmed by local proven. The medication scheme will be directly compared with the Cordonzumab combined chemotherapy scheme. The exploration scheme includes: T-DXD single medicine, T-DXD combined chemotherapy, T-DXD combined immunity, T-DXD combined chemotherapy and immunity.
Figure 13. Destiny-Gastrid 03 Study [23]
Results show that [24], HER2-positive gastric cancer patients who have progressed after the treatment of the Quchozumab solution can continue to benefit from T-DXD combined chemotherapy, T-DXD combined with 5-FU group ORR up to 66.7%, T-DXD combination The CAP group Orr reached 71.4%, and the PR2D was T-DXD 6.4mg/kg+5-FU 600mg/m2, T-DXD 6.4mg/kg+Kaibibin 1000mg/m2.
03
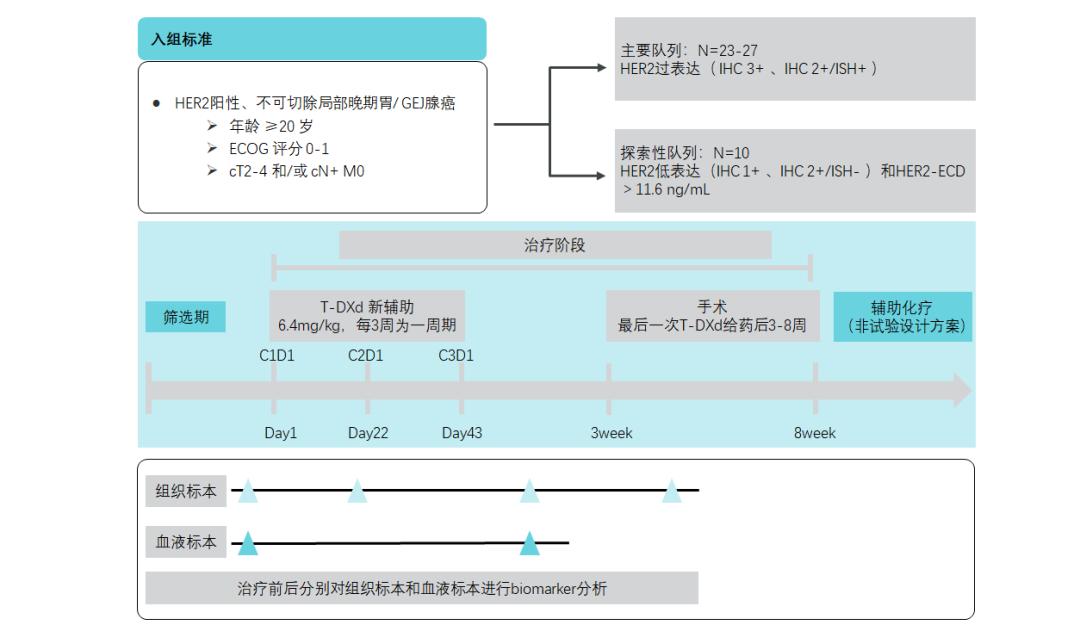
Entry Xinfu! T-DXD wants to move from late to early
EPOC2003 Research-T-DXD exploration of early gastric cancer
In addition to the advanced front movement, clinical research is also exploring T-DXD as a plan for early new auxiliary treatment. EPOC2003 studies the anti-tumor activity of T-DXD neo-assisted treatment among HER2-positive gastric cancer patients [25]. At present, there are other anti-HER2 treatment explorations in the treatment period of perioperative surgery.
Figure 14. EPOC2003 Research Design [25]
Summarize
The A-J101 study initially explored the efficacy and safety of T-DXD single drugs in patients with advanced gastric cancer in HER2. Destiny-Gastrid 01 Study based on the control of chemotherapy confirmed that T-DXD excellent efficacy among these groups of people And security. At the same time, T-DXD also has a registered research layout in the late first and second lines. The scheme covers T-DXD single medicine and a joint solution with T-DXD as the core. In addition, a research initiated by a researcher will explore the efficacy and safety of T-DXD single drugs to patients with HER2 positive gastric cancer during the new assistant stage.
According to Destiny-Gastrid 01 studies, T-DXD has obtained the approval of Japan (MHLW) and the United States (FDA) for back-line therapy for HER2-positive gastric cancer patients, and also wrote the "CSCO gastric cancer diagnosis and treatment guide" in 2022. It is believed that the successive announcement of T-DXD research results in the future will provide more treatment options for patients with HER2 positive gastric cancer.
Expert Introduction
Professor Bai Chunmei
Beijing Union Hospital of the Chinese Academy of Medical Sciences, director of the Department of Internal Medicine
Director of the Council of the China Clinical Oncology Society
Chairman of the China Clinical Oncology Society of Neurological Endocrine Oncology Expert Committee
Executive Member of the Head and Neck Oncology Expert Committee of the China Clinical Oncology Society
Executive Member of the Smart Medical Expert Committee of the China Clinical Oncology Society
Deputy Chairman of the China Medical Promotion Association's Neurological Endocrine Tumor Branch
Executive member of the Society of Old Cancer Society
Executive Member of the Professional Committee of Pancreatic Diseases in China Research Hospital
Fields are good: early research and development of new anti -tumor drugs; tumor diagnosis and treatment, especially molecular markers in tumor diagnosis, treatment and prognosis
references:
[1].Bartley AN, Washington MK, Colasacco C, et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017 Feb; 35 (4): 446-464.
[2] .janjigian yy, kawazoe a, yañez p, et al. Keynote-811 trial of dual PD-1 and her2 blockade in her2-positive gastric car. Nature. 2021 dec; 600 (7890): 727-7300 (7890): 727-7300 (7890): 727-730 (7890): [3].Xu Z, Guo D, Jiang Z, et al. Novel HER2-Targeting Antibody-Drug Conjugates of Trastuzumab Beyond T-DM1 in Breast Cancer: Trastuzumab Deruxtecan(DS-8201a) and (Vic-)Trastuzumab Duocarmazine (SYD985 ). EUR JMED CHEM. 2019 DEC 1; 183: 111682.
[4].Nishida Y, Kuwata T, Nitta H, et al. A novel gene-protein assay for evaluating HER2 status in gastric cancer: simultaneous analyses of HER2 protein overexpression and gene amplification reveal intratumoral heterogeneity. Gastric Cancer. 2015 Jul;18 (3): 458-66.
[5] .Yao x, jiang j, Wang x, et al. A Novel Humanized Anti-Her2 Antibody Conjugated with Mmae Exerts Poti-Tumor Activity. Breast CANCER Res Treat. 153 (1): 123-33.
[6].Nakada T, Sugihara K, Jikoh T, et al. The Latest Research and Development into the Antibody-Drug Conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem Pharm Bull (Tokyo ). 2019; 67 (3): 173-185.
[7].Skidmore L, Sakamuri S, Knudsen NA, et al. ARX788, a Site-specific Anti-HER2 Antibody-Drug Conjugate, Demonstrates Potent and Selective Activity in HER2-low and T-DM1-resistant Breast and Gastric Cancers. Mol Cancer Ther. 2020 SEP; 19 (9): 1833-1843.
[8]. Tsuchikama K, An Z. Antibody-Drug Conjugates: Recent Advances in Conjugation and Linker Chemistries. Protein cell. 2018 Jan; 9 (1): 33-46.
[9].Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin Cancer Res. 2016 Oct 15; 22 (20): 5097-5108.
[10] .zhang w, Wang S, Wang Q, Yang Z, PAN Z, Li L. Overexpression of Cadeine Cathepsin L is a Marker of Invasion and Metastasis in Ovarian Cancer. Oncol (31 (3): 1334 -42.[11].Marcoux J, Champion T, Colas O, et al. Native mass spectrometry and ion mobility characterization of trastuzumab emtansine, a lysine-linked antibody drug conjugate. Protein Sci. 2015;24(8):1210- 1223.
[12] .Conilh L, Fournet G, Fourmaux E, et al. Exatecan antibody dragConjugates base
[13] .zhang j, ji d, shen w, et al. Phase I trial of a Novel Anti-Her2 Antibody-Drug Conjugate, ARX788, for the Treatment of Her2-POSITASTASTASTASTASTASTASTASRR. Clin cancer RES. 20222222222222222222222222222222222222222222222222222 : OF1-OF10.
[14].Krop IE, Beeram M, Modi S, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010 Jun 1; 28 (16): 2698-704.
[15].Nagai Y, Oitate M, Shiozawa H, et al. Comprehensive preclinical pharmacokinetic evaluations of trastuzumab deruxtecan (DS-8201a), a HER2-targeting antibody-drug conjugate, in cynomolgus monkeys. Xenobiotica. 2019 Sep;49(9 ): 1086-1096.
[16].Shitara K, Iwata H, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol. 2019 Jun;20( 6): 827-836.
[17].Shitara K, Bang YJ, Iwasa S, et al; DESTINY-Gastric01 Investigators. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med. 2020 Jun 18;382(25):2419-2430.
[18].Trastuzumab deruxtecan (T-DXd; DS-8201) in pati ents with HER2–positive advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: Final overall survival (OS) results from a randomized, multicenter, open-label, phase 2 study (DESTINY-Gastric01). 2022 ASCO GI Abstract. 242.[19].Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-low, advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: Results of the Exploratory cous in the phase II, multicenter, Open-Label Destiny-Gastric01 Study. 2020 ESMO Mini Oral 1422.
[20] .https://clinicaltrics.gov/ct2/show/nct04989816
[21].Primary Analysis of a Phase 2 Single-Arm Trial of Trastuzumab Deruxtecan (T-DXd) in Western Patients With HER2-Positive (HER2+) Unresectable or Metastatic Gastric or Gastroesophageal Junction (GEJ) Cancer Who Progressed on or After a Trastuzumab -Containing regimen.2021 esmo.abstract LBA55.
[22] .https: //Clinicaltribe
[23] .https: //Clinicaltrics.gov/ct2/show/nct04379596
[24].Dose-escalation and dose-expansion study of trastuzumab deruxtecan (T-DXd) monotherapy and combinations in patients (pts) withadvanced/metastatic HER2+ gastric cancer (GC)/gastroesophageal junction adenocarcinoma (GEJA): DESTINY-Gastric03. 2022 Asco gi. Abstract 295.
[25].Phase 2 study of trastuzumab deruxtecan in the neoadjuvant treatment for patients with HER2-positive gastric and gastroesophageal junction adenocarcinoma (EPOC2003). 2022 ASCO. Abstract TPS4161.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
Lan Fan Yue Reading 丨 Pingliang Forest Fire Detachment continues to lead the "six reading" reading activities to deepen

Life is full of weather because of reading. Reading can clear the meridians of the...
Oregon Track and Field World Championships Su Bingtian missed the men's 100 -meter final

Xinhua News Agency, U.S. News Agency, July 16th. In the men's 100m semifinals in t...