Regarding the EGFR EX20INS mutation NSCLC, which new drugs have made new progress?丨 2022 asco
Author:Cancer Channel of the Medical Time:2022.06.16
*For medical professionals for reading reference

Latest research progress
The annual conference of the American Clinical Oncology Society (ASCO) was held on June 3-7, 2021 with an online virtual conference situation. At the meeting, the treatment of non -small cell lung cancer (NSCLC) ushered in many new progress. In this article, the EGFR 20 exogenous sub -insertion (EX20INS) mutation NSCLC treatment related studies to readers to readers.
1
CLN-081 Treatment of EGFR EX20INS mutations NSCLC patients are safe and effective
CLN-081 is a new, irreversible, oral EGFR tyrosine kinase inhibitor (TKI). It has extensive activity for EGFR mutations including EX20Ins, and the selectivity of EX20INS is higher than the wild type (WT) EGFR. CLN-081 has been determined by the Breakthrough of the US Food and Drug Administration (FDA) for the treatment of patients with EGFR EX20Ins NSCLC.
A multi-center I/IIA study was included in patients with recurrence or metastatic NSCLC patients carrying EGFR EX20INS mutations, including patients with brain metastases with a stable condition ≥4 weeks, and evaluated the safety and efficacy of CLN-081. (Summary number: 9007)
The results showed that 73 patients were included. 66%of patients' past treatment lines ≥2, 36%of patients were treated with EGFR-TKI, and 55%of patients received immunotherapy.
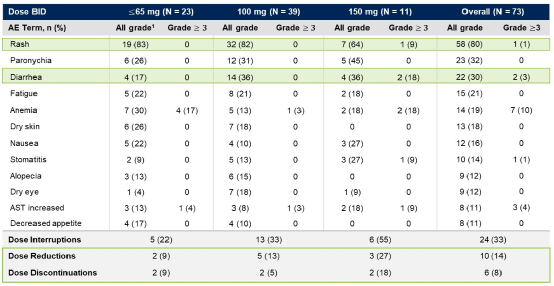
The dose range of the CLN-081 is 30 ~ 150 mg BID. Most adverse events (AE) are level 1/2; when the dose of CLN-081 is <150 mg, the dose is reduced and the drug discontinuation is rare, and level 3 rash and diarrhea are not observed (Table 1).
Table 1 Treatment related adverse events
Among the overall crowds, 28 cases (38.4%) confirmed the part of (PR), 42 (57.5%) disease stable (SD), and 3 (4.1%) disease progress (PD). In the sub -group of 100 MG BID patients, the total relief rate (ORR) was 41%, and the mid -bit relieving duration (MDOR) & 21 months (Figure 1), MPFS for 12 months (Figure 2).
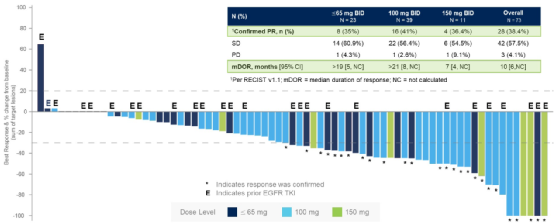
Figure 1 The best percentage change of the target lesion from the baseline and the confirmation and relief rate of the hierarchical hierarchy of the CLN-081 dose
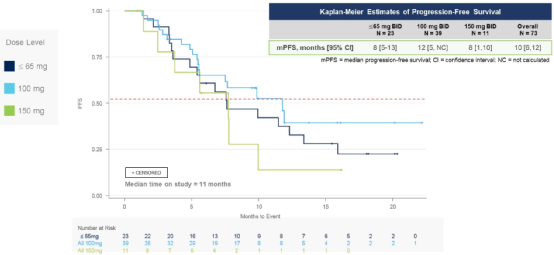
Figure 2 Analysis of no progressive survival analysis according to the dose level
The results show that the safety characteristics of the CLN-081 dose of <150 mg BID can stand the test. For these patients who have received a lot of preparations, including other patients with EGFR-TKI treatment, they can get objective relief from CLN-081 treatment, especially the effect of the dose when the dose is 100 mg BID is very encouraged.
2
For the treatment of EGFR EX20Ins+ metastasis NSCLC patients, Mobocertinib research results are released
Mobocertinib is an EGFR EX20INS that is effective, irreversible, oral TKI, which can selectively target NSCLC.
A open label and multi -center study in 114 patients with EGFR EX20Ins+ MNSCLC (PPP) patients (PPP), analyzed data from the first PD part (brain and external) to continue Mobocertinib in PPP queue to treat patients with patients with patients with patients with PPP queues. Essence (Summary number: 9099)
The results showed that the Objective Relief rate (CORR) of the Independent Examination Committee (IRC) evaluation was 28%and the MDOR was 17.5 months. The IRC assessment of the IRC evaluation of the inferior brain metastases is 34%, while the patients with baseline brain metastasis are 18%; the median no progressive survival (PFS) of the two is 9.2 months and 3.7 months, respectively (Table 2).
According to the evaluation of researchers (INV), 64 patients were PD. Among the 21 patients with the first PD part of the PD part (11 of them were involved in the brain), 17 cases (81%) PD continued to use Mobocertinib for treatment (Figure 3); 7 cases (33%) After receiving brain radiotherapy and continuing Mobocertinib for treatment, 3 of them continued to use Mobocertinib for ≥6 months, and 1 case was ≥ 12 months. Among the first patients with an extra -skull (43 cases), 28 cases (65%) continued Mobocertinib for treatment after PD, and 4 cases (9%) continued Mobocertinib for ≥6 months.
In view of the high frequency of the brain (25%) of the first PD part, IRC evaluation CORR in patients with baseline brain metastases is lower in value. As a result, Mobocertinib may have limited intracranial activity. However, patients with PDs that affect the brain may benefit from Mobocertinib for combined radiotherapy (treating brain lesions).
Table 2 The overall queue and Mobocertinib, which is made of unsecured brain metastasis
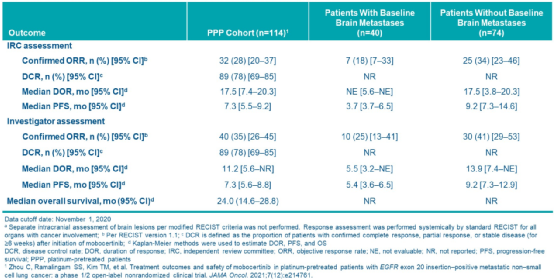
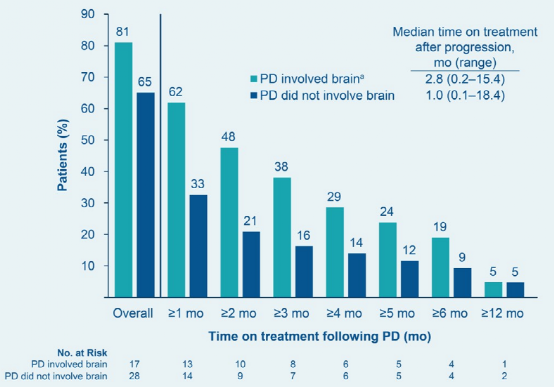
Figure 3 follows the first disease progress of the disease, and the proportion of patients treated with Mobocertinib continued to use Mobocertinib
3
For patients with EGFR EX20ins NSCLC, the effects of Mobocertinib and amivantamab are similar to Mobocertinib (MOBO) and AMIVANTAMAB (AMI). They have been approved by the US FDA for local advanced or metastatic NSCLC patients in the treatment of platinum chemotherapy.
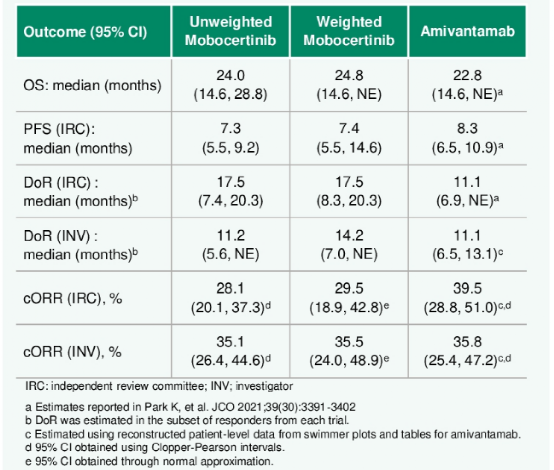
For a phase I/II single -arm research (MOBO 160 mg QD, N = 114) and a phase I phase single -arm study [AMI 1050 mg (1400 mg, ≥80 kg), n = 81] Platinum category in the medium category For patients with EGFR EX20Ins+ NSCLC, the researchers used the non -anchored MAIC method to compare CORR between MOBO and AMI, alleviating duration (DOR), PFS, and total survival (OS). (Summary number: 9115)
The results showed that after matching and adjusting indirect comparison (MAIC) weighted (MAIC), MOBO treatment of baseline characteristic balance between patients and AMI treatment patients.
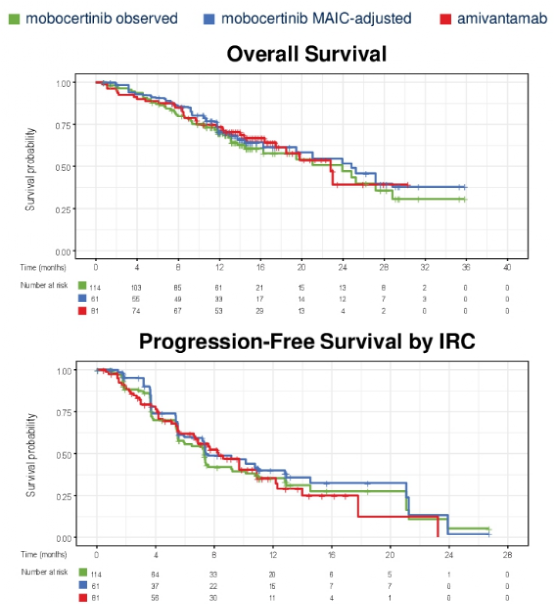
For the OS and Corr evaluated by INV, MOBO therapy patients are similar to AMI treatment patients (Table 3, Figure 4).
For CORR evaluated by IRC, AMI therapy patients are higher in value (or = 0.64, P = 0.230). For IRC PFS, the risk ratio (HR) is beneficial to MOBO in terms of value (HR = 0.82, P = 0.417).
Among the respondents, MOBO is more DOR (INV evaluation DOR: HR = 0.44, P = 0.049; IRC evaluation DOR: HR = 0.56, P = 0.149).
Overall, MOBO and AMI seem to have similar effects. At the same time, the two drugs have different mechanisms and dosage channels, which can provide different options for the treatment of EGFR EX20In+ NSCLC.
Table 3 MAIC's treatment effect before and after weighted
Figure 4 General survival and no progressive survival curve
4
For patients with EGFR EX20Ins+late NSCLC, CTDNA molecular analysis helps to evaluate the state of mutation and disease progress
Circular tumor DNA (CTDNA) is an important tool for diagnosis and monitoring gene mutations in patients with NSCLC. For an EGFR EX20Ins+late NSCLC patients treated with Mobocertinib (160 MG QD) in phase I/II studies, researchers evaluated the EGFR EX20INS mutation of tumor and plasma samples, analyzed the gene frequency of EGFR EX20Ins after mobocertinib the treatment frequency, (VAF) change and its correlation with the treatment response, and identify the potential new variation of obtaining drug resistance. (Summary number: 9108)
In the baseline, the researchers collected tumor tissue samples; plasma samples were collected at the baseline, 2 months, and treatment.
The results showed that 29 patients (76%) BL were detected by CTDNA in 38 patients of 38 organizational examinations to confirm the EGFR EX20Ins+ NSCLC patients. Among PR or SD patients after MOBOCERTINIB were treated, the VAF of EGFR EX20Ins was significantly reduced during the treatment of 2 months, but there was no in PD patients.
In the 127 genes of 33 patients, 217 mutations were detected. EGFR, TP53, and DNMT3A were the most common genes for mutation. Among them, new mutations were observed among the two mutations hot spots of EGFR 20 outer sons (Figure 4), including mutations (5 patients) on the 6 "5 patients), 5 T790M goalkeeper mutations ( 5 patients).
The results showed that when the BL tissue and the plasma CTDNA mutation was 76%. For PR and SD patients treated with Mobocertinib, the EGFR EX20INS VAF has dropped significantly after 2 months of treatment, but there is no in PD patients. New mutations are often observed at the EGFR insertion sites (after α C spiral) and goalkeeper mutation T790M. The new EGFR mutations appearing at the T790M on the ring C -spiral may be resistant to the Mobocertinib.
Fig

5
Veridinib the treatment of Chinese EGFR EX20INS mutations NSCLC patients at the beginning
A Chinese study retrospectively observed the short -term efficacy and safety of Faminini in the Chinese EGFR EX20INS mutant NSCLC patients. The study retrospectively collected 15 patients with EGFR EX20INS mutations NSCLC patient information in the two medical institutions in Changzhou, Jiangsu Province from April 2021 to December 2021; these patients were treated with oral ildini 160mg for orally, until they were treated. Progress of disease, drug intolerance, patients refused to use medicine or death. (Summary number: E21063)
The results showed that the number of past treatment lines of patients was ≥2, the median age was 62 years old, and men accounted for 53.3%(8 cases). The ECOG score was mostly 1 point (13 cases, 86.7%). , 86.7%). Among the 15 patients, the researchers discovered 10 different EGFR EX20INS mutations, of which P.A767_V769DUP (3 cases) and P.A767_v769Dup (4 cases) were more common.
At the end of the follow -up, 15 patients completed at least once at least one curative effect, and tumor fascinating to varying degrees. 8 patients reached PR and 7 patients were SD. ORR is 53.5%, and the disease control rate (DCR) is 100%. The 3 -month PFS rate is 100%.
In addition, level 3 or above AE was observed.
The results show that the third-generation EGFR-TKI Vamidini has good curative effect and acceptable security. In view of the heterogeneity of the EGFR EX20INS mutation, the efficacy of targeted therapy may vary depending on the type of mutation, and further research is needed.
references:
[1] Helena Alexandra Yu, et al. Phase (pH) 1/2A Study of Cln-081 in Patients (PTS) with NSCLC With EGFR EXON 20 Insertion Mutations (INS20). 2022 asco Abstract #9007.
https://meetings.asco.org/abstracts-prencentations/208973
[2] PASI A. Janne, et al. Mobocertinib (TAK-788) in EGFR EXON 20 Insertion (ex20ins)+ metastatic non -Small cell (Mnsclc): Treatment (TX) PROGRESERESERESERED) pretreated patients (PTS) with and withtra crocranial pd. 2022 asco abstract #9099.
https://meetings.asco.org/abstracts-preentations/212854
[3] Sai-Hong Ignatius Ou, et al. Matching-adjusted indirect comparison (MAIC) of mobocertinib versus amivantamab in patients with non–small cell lung cancer (NSCLC) with EGFR exon 20 insertions (ex20ins). 2022 ASCO Abstract #9115 Then, then, then
https://meetings.asco.org/abstracts-prencentations/212874
[4] Sylvie Vincent, et al. Molecular analysis of circulating tumor DNA (ctDNA) in patients (pts) with EGFR exon 20 insertion-positive (ex20ins+) advanced NSCLC treated with mobocertinib. 2022 ASCO Abstract # 9108.
https://meetings.asco.org/abstracts-prencentations/212964
[5] xiaoyue zhou, et al. SHORT-TERM EFFICACYACY of FurMonrtinib in Treatment of NSCLC Patients with EGFR EXON20 Insertion. 2022 ASCO ABSTRACT # E21063.
https://meetings.asco.org/abstracts-prencentations/208133
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
It's so happy to play with water like this!

On June 26, the gardener of the garden and rural complex in Shaliangzi Village, Ch...
Leling promotes regional evaluation results to transform | A guide for the project to start 40 days in advance

In the past, the project in the park required a professional soil and water conser...