New story grows in the PD-1 arena, and the domestic counterattack is being staged
Author:Kenji Bureau Time:2022.06.08
In the early summer season, the Annual Meeting of the American Clinical Oncology Society (ASCO) is a arena for global innovative pharmaceutical companies.
ASCO has announced hundreds of research results over the years, and not every study can get the opportunity to show. It is already a big breakthrough to be able to obtain a wall newspaper display. The opportunity for oral reports on the spot must be left to the most innovative varieties.

Since then, H medicine has become the first domestic PD-1 in the field of first-line lung cancer.
In China's fiercely competitive PD-1 market, Fuhong Hanlin did not grab the first advantage. It was not until March 24 this year that H medicine was approved in China and became the 7th domestic PD-1.
Late admission, but was recognized by the industry. Why? The answer is three words: differentiation.
\"FDA or EMA, definitely recognizing data. Ji Lin, Director of the Second Section of Hunan Cancer Hospital and one of the clinical researchers in H medicine, believes that this is the key to ASTRUM-005 clinical research on ASCO.
Although it is called \"universal anticancer drug\", no PD-1 has been made in the indications of small cell lung cancer. In December 2020 and March 2021, the two major ace of the O -medicine and K medicine have proactively withdrawn the application for the indications of small cell lung cancer, announcing the failure of clinical trials.
Until now, global PD-1 is still blank in the field of small cell lung cancer. The industry also doubted: Is small cell lung cancer really a penalty area of \u200b\u200bPD-1?
The results of the ASTRUM-005 clinical research gave the answer and displayed to the world on an occasion like ASCO.
In a sense, Astrum-005 improved the clinical practice level of domestic anticancer drugs.
From the beginning of the design, Fuhong Hanlin has communicated with FDA, EMA, CDE and other institutions at the same time. This is because the sample volume of ASTRUM-005 is very large-585 subjects were screened in 114 clinical centers worldwide, of which about 31.5%were Caucasia.
Why do you determine such a complex and high -risk clinical research on the horse? Zhu Jun, President of Fuhong Hanlin, told Jianxian Bureau to do innovative medicines along the most simple method.If there is something wrong, that is: the most reliable data results are obtained with the most sufficient test.

Since 2022, Cinda Bio, Hetai Medicine, Junshi Bio and other pharmaceutical companies Fasting to the sea, setting off a major discussion:
March, the FDA believes that the clinical trial of Cinda PD-1 Xindi Lili Anti-Anti-Anti-Anti-Anti-Anti-Anti-Anti-Anti-Anti-Anti-Anti-Anti-Anti-Anti-Anti-Anti-Anti-Clinical Test. Approval; in May, the application for the listing of the listed on the listing of Tiffini in the Sofi Pharma is delayed and needed to make the international multi-center test; or in May, the Junshi PD-1 Tripley monoclonist also went to the sea to meet. Change a quality control process.
The design of ASTRUM-005 is the two points that the above-mentioned overseas regulatory agencies are most concerned: the degree of clinical multi-center is sufficient and the research and development direction is differentiated.
In various occasions that Chinese innovative pharmaceutical companies communicated publicly, the executives of Fuhong Hanlin repeatedly emphasized differentiation. In fact, the first indication of H drugs approved in China is that the high -satellite instability physical tumor is an unpopular direction. Later, I saw the indications of small cell lung cancer, which was also because it was difficult to give Os and K drugs abandonment in this field. Therefore, few companies were involved in, and there were few persistence all the way.
In the end, for the clinical trial results of H drugs on small cell lung cancer indications, Zhang Wenjie, chairman of the company, executive director and CEO of the company, said, \"Fu Hong Hanlin's PD-1 is differentiated Fortunately, the lucky data has given us the natural differentiation ability. \"
For Chinese innovative pharmaceutical companies, such differentiated indications layout is more conducive to products to go to sea.
Vision from \"Stars\"
People in the industry can clearly see that such product design strategies mean At the beginning, he was planning to go to sea.
The Jianxian Bureau learned that H medicine has obtained clinical trial permits in China, the United States, Europe and other countries and regions. In the global group, more than 2,800 people are admitted to the world. It is a PD-1 product with more international clinical data one.
ASTRUM-005 results show that the median total survival of the general period of small cell lung cancer in the first-line treatment of H drug combined with chemotherapy is 15.4 months, and the total survival rate of two years is 43.1%.
What is this concept? In comparison, in this indication, the survival rate of simplicity of chemotherapy is only 5%-6%, and the data announced by the listed PD-L1 products is about 20%.
In Latin, Avrum refers to \"stars\". Fu Hong Hanlin hopes that H medicine can bring hope to patients like stars.
Fu Hong Hanlin has a research center in Shanghai and California, and the two centers complement and shared, forming a good interaction. ReunionHanlin also formed a global product development team of more than 300 people to build a new clinical operation and drug registered system. In this regard, Zhu Jun believes that the unsatisfactory medical needs must be focused on the early stages.
In addition to the indications that have been listed, the indications for H drug declaration also include ingredients non -small cell lung cancer, first -line treatment of small cell lung cancer, mainly in liver cell carcinoma, esophageal cancer, head and neck cancer, gastric cancer, etc. Tumor species also have layout.

In April 2022, the indication of the lung cancer of the Hidalial small cell lung cancer obtained the FDA's orphanage qualification identification ; China Clinical Oncology Society of Small Cell Cancer Cancer Diagnosis and Treatment also regards H drugs combined with chemotherapy as a wide range of SCLC treatment (Class 1).
Marketization has long been layout
At present The four products of the four products of Tushu Mipuke, Ada Ming Ming Ming and Bayu Ming Midurate were approved for listing. Among them, Tuskuzumab was also listed in China and the European Union at the same time.
And H medicine became the first biological innovation medicine listed on Fuhong Hanlin. Fuhong Hanlin had planned for a long time for its commercialization.
In China, Fuhong Hanlin has formed a sales team of more than 200 people for H medicine. Zhang Wenjie believes that H medicine has important strategic significance for Fu Honghanlin, so there must be a special commercial team. At present, Fuhong Hanlin has established an independent commercial team of more than 800 people on the domestic market, responsible for the commercialization of the company's core tumor products.
In the 12 years since its birth, as an innovative pharmaceutical company, Fuhong Hanlin has been doing two things: building an international R \u0026 D platform and quality system, and accumulating rich product pipelines. Today, Fuhong Hanlin is evolving from a Biotech to Biopharma. H medicine is the key product in evolution.
In fact, the name \"H medicine\" shows the confidence of Fu Hong Hanlin. It is hoped that it can become a PD-1 with international quality like K medicine and O medicine.
At present, China's innovative pharmaceutical industry has entered a new round of adjustment period and returns to the real hard core innovation attributes. In the PD-1 arena, K medicine has staged a wonderful competition for counterattack O. Today, another good show belonging to domestic innovation has just appeared on stage.
The story of PD-1 is not over.
#Innovative medicine ## PD-1#
- END -
Multiple measures and nursing nursing entrance examinations, Guilin Qixing City Management fully guarantees the city's capacity environment

On June 24, the third students entered the entrance examination room. In order to ...
Out of scores!Score line delineation, admission work arrangement ...
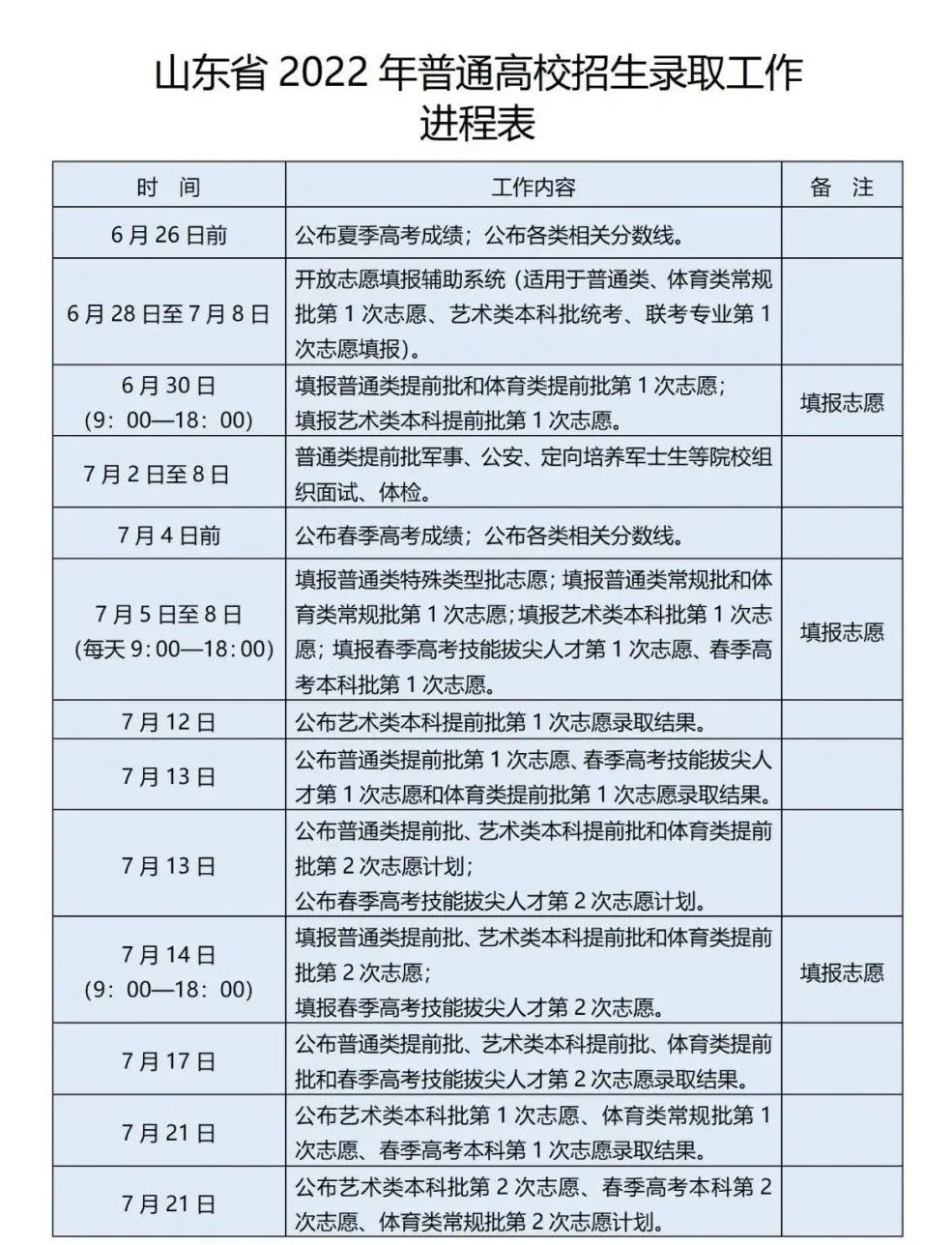
June 25 16:00The second press conference of the summer college entrance examinatio...