"Wang Fried" combination of folding halberd liver cancer, domestic PD-1 combination therapy ushered in new opportunities
Author:Yaizhi.com Time:2022.08.10
"Wang Fried" combination of folding halberd liver cancer, domestic PD-1 combination therapy ushered in new opportunities
Source: Yaozhi.com/Wilson
As a great country B, the incidence of liver cancer, especially liver cell carcinoma in my country, has always been higher than other countries and regions in the world. However, because the treatment of liver cell carcinoma has no good chemotherapy drugs to choose from, the main method of treatment of liver cell carcinoma that cannot be surgically removed currently depends on targeted therapy and immunotherapy. Among them, Multi-receptoricase kinase inhibitors Lenvima and KEYTRUDA (Keytruda) of anti-PD-1 are two typical representatives in targeted and immunotherapy in liver cell carcinoma. There is a clinical trial that the single medicine treatment of these two medicines is effective for patients with liver cell carcinoma.
So, what is the effect of Lenndininib and Paborzab's treatment of liver cell carcinoma?
Recently, the data of Phase III clinical trial Leap-002 compared to the comparison of Lenndininib and Lenvarini single drugs in Pabberzumab is surprising. Patients who have been treated with Lenvarini single drugs (PFS) are treated with no progressive survival (PFS), but the degree of improvement has not achieved significant differences.
Source: Merha East Official Website
What is the reason for the "Wang Fried" combination therapy?
LEAP-002 is a multi-center, random, and double-blind phase III clinical trial. The purpose of the experiment is to evaluate whether the efficacy of the treatment of patients with liver cell carcinoma in the treatment of liver cell carcinoma Essence This experiment was included in 750 patients, and patients were HCC patients who could not surgical resection. They were randomly paid at a ratio of 1: 1 to receive two different treatments. However, it is worth mentioning that the total survival period of the patients in the treatment of patients in the clinical trials is more than the treatment of the treatment of Lenami Noni drug treatment in the previous clinical trials, reminding the admission patients and the condition and the situation of the patients and the admission patients and the situation and the condition of the patients and the situation of the patients and the situation and the condition of the patients and the situation and the situation of the patients and the situation and the situation of the patients and the situation of the patients and the situation and the situation of the patients and the patients of the patients and the situation and the condition of the patients and the patients of the patients and the situation and the situation of the patients and the patients and the situation of the patients and the patients and the patients and the patients and the patient's condition and the situation and the situation of the patients and the case of the patients and the situation of the patients and the situation of the patients and the patients and the patients of the patients. In the past, clinical trials were slightly different, and more detailed clinical data will be displayed at a recent medical conference.
Dr. Gregory Lubiniecki, vice president of Global Clinical Development of the Meridodon Research Lab, said: "Keytruda combined with Lenvima therapy is committed to providing a treatment method for those patients with the most difficult cancer (such as liver cell carcinoma) to meet the needs of these patients." "According to a large amount of evidence we see so far, we are still confident in the potential of this combination, and will continue to study the treatment of two drugs in other types of cancer."
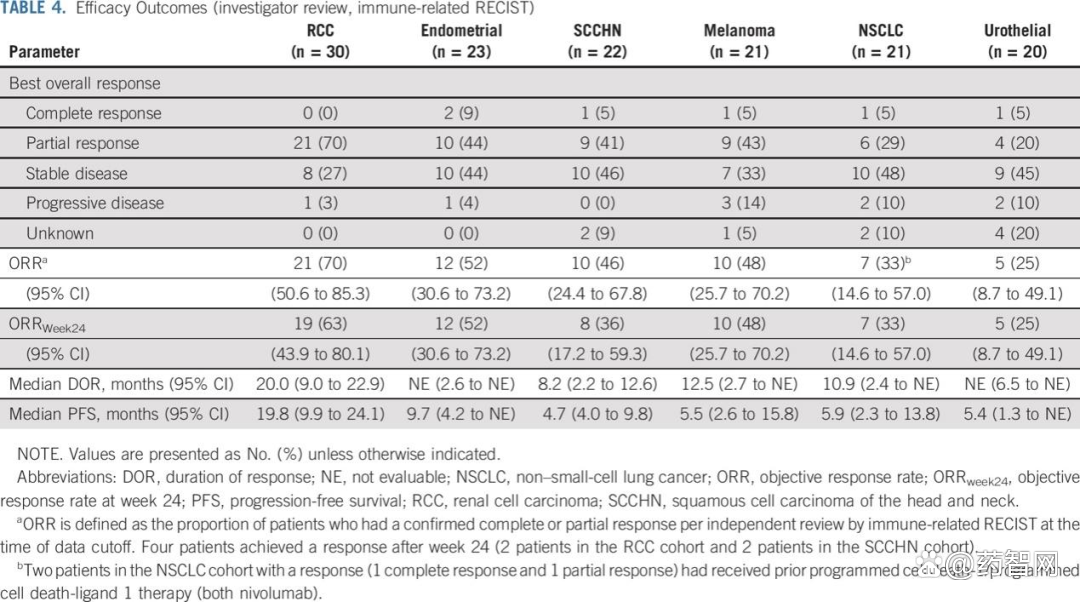
In fact, Lenvima and KEYTRUDA combined therapy have proved other powerful treatment effects in many different cancers, including kidney cancer, endometrial cancer, non -small cell carcinoma carcinoma A number of cancers such as head and neck squamous carcinoma, melanoma, and urinary tract cancer have significant effects (see the figure below). Because of this, the combined therapy of the two is called the "king bombing combination" and "cola combination".

Source: Reference Information 1
However, the Wangwan group is not a panacea. Except for the loss of this LEAP-002 clinical trial, in 2021, the Leap-007 clinical trial in the field of non-small cells in the stage of Non-small cells in stage III. None of the expected clinical benefits.
However, in this LEAP-002 clinical experiment, the same effectiveness of the same treatment scheme of Lenvima and KEYTRUDA in different cancers is actually a deeper deepening Thinking. On the one hand, the combination of Lenvatinib and Paborizumab joint treatment is effective in many different cancers, indicating that there are some commonality between different tumors, such as the occurrence of different cancer species and the development process. Participation, this is also an effective mechanism basis for immunotherapy in multiple cancers. On the other hand, the combined treatment of the two drugs in different cancers is the difference in efficiency in different cancers. It shows that for different cancer species, it is still necessary to formulate more individualized treatment solutions based on their own characteristics.
From the perspective of liver cell carcinoma, articles have speculated that the cause of the cause is an important factor affecting the therapeutic effect of liver cancer. The cause of HBV etiology can benefit from the treatment of "Immune + TKI", while the HCV causes liver cancer has limited benefits from "immunity + TKI".
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infection is a common risk factor for liver cancer in my country, especially chronic HBV infections. About 90%of patients in China have HBV infection history of HBV infection. Although LEAP-002 is folding liver cancer, immune combined therapy still has great potential in the treatment of liver cancer, especially the chances of clinical research based on Chinese patients are relatively large.
For example, the "Double Ai Combination" (Erica and Aitan) is the world's first combination to announce the success of the "PD-1 + TKI" strategy.
PD-1+TKI domestic group ushered in the opportunity to go to the sea
In fact, the PD-1+TKI use plan is the main treatment plan for metastatic liver cancer in the CSCO guide.
In the 2022 version of the liver cancer diagnosis and treatment guidelines, the "Shuang Ai combination" was upgraded from the first -line treatment plan in the 2020 guide to one of the preferred treatment plans. Although the first -line treatment of the Shuang Ai combination has not been officially approved, the first -line recommendation has been obtained. It can be seen that the research therapy that has outstanding curative effects and can be recognized by authoritative experts can be written in advance. Essence In May this year, Hengrui announced the "Double Ai" plan International Multi-Center III (SHR-1210-Ⅲ-310) to reach the main end point. The specific data has not yet been announced. However, from the stage of II -Rescue Studies, it can be seen that the Shuang Ai group MOS is 20.1 months, the 12-month OS rate is 68.3%, the MPFS is 5.7 months, the ORR is 34.3%, the CR is 1.4%, the DCR is 77.1%(77.1%( Rescue, the incidence of level 3 TRAE is 77.4%, showing good anti -tumor activity and controllable safety in the front line of liver cancer treatment.
At present, the first -line indicator of the plan of the plan has been accepted by the China State Drug Administration. At the same time, Hengrui plans to submit a listing application to the FDA, which is expected to become an important milestone for Hengrui to innovate.
Table 1 2020 and 2022 CSCO late HCC front -line treatment strategy selection comparison
Source: According to Hua'an Securities Information, Organization

In addition, clinical studies in China have a number of PD-1+Lenvetinib to treat liver cell carcinoma. Among them, the Tripley Mippitine of the Junshi Bio+Lelbutoni first -line therapy for advanced liver cell carcinoma NCT04523493 (data read out in 2023), the Schogly Mippitine of the cornerstone pharmaceutical, the first line of Lenvarini to treat advanced liver cells Cancer NCT04194775 is carried out clinical research on the third phase of the International Multi -Center. If it is successful, it will have global influence.
Joint treatment is currently a major trend of anti -tumor treatment, but the combined treatment of drugs is the problem of simple 1+1 = 2 or 1+1> 2> 2. The final treatment effect of combined treatment is the result of the interaction between many factors such as the mechanism of different drugs, tumor microenvironment, and cause of tumor. It is good for patients.
Although Lenndinib+Paborzab resistant to folding liver cancer, from the perspective of many previous clinical experimental data, combined therapy based on PD-(L) 1 is still worth looking forward to.
参考资料:1. Taylor, Matthew H., et al. "Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors." Journal of Clinical Oncology 38.11 (2020 ): 1154.2. LEAP-002 folding halberd liver cancer, the cause is closer to the truth?
- END -
Shanghai yesterday added 1 local confirmed cases and 4 symptoms of local infections

The Municipal Health and Health Commission notified this morning (July 30): At 0-2...
In 2022, Zhang Yongze, Zhang Yongze, was doubled

At 11:00 on July 7, the website of the Central Commission for Discipline Inspectio...