After EGFR-TKI drug resistance, MET amplified lung cancer-brain metastase patients, Cizinininininini brings lasting benefits
Author:Cancer Channel of the Medical Time:2022.08.12
*For medical professionals for reading reference

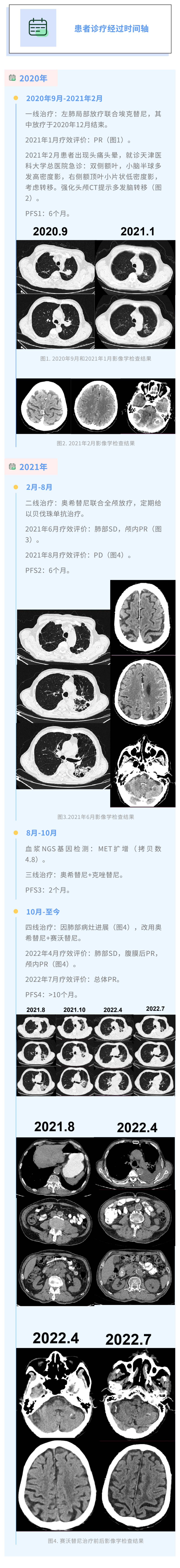
A period of EGFR mutant patient treatment journey.
This case is a patient with multi -hair lymph nodes (IIIB, T3N2M0) patients with mutant L858R (21L858R) mutation of EGFR 21 (21L858R). Hitinib+Bevarzumab+full skull radiotherapy second -line treatment. After 6 months, the patient's lung lesions progressed, and genetic testing showed MET amplification. Patients were treated with Oshitinib+Kizibinib, but after two months, they found that the development of the lung lesions was progressing, so it was treated with Oshitinib+Savidininib. In April 2022, the efficacy evaluation showed that the lung lesions maintained disease stability (SD), part of the postcard lesion partial relief (PR), and intracranial lesions PR; in July 2022, the efficacy evaluation was evaluated as the overall PR. So far, patients have obtained the non -progressive survival (PFS) of more than 10 months, and they are still benefiting. The case was provided by Professor Zhang Weihong, a Tumor Hospital of Tianjin Medical University, and invited Professor Ren Xiubao of Tianjin Medical University Cancer Hospital for review.
Profile
▎ Basic situation
Male, 71 years old.
In May 2020, the patient was diagnosed with a three -level hospital in Tianjin due to discomfort in his heart.
After admission, chest CT examination: Multiple soft tissue tissue tissue on the left lungs, the size is 2.9cm × 1.9cm. PET-CT: Multi-cubes on the left lung leaf, consider multi-center lesions. It is recommended to treat thoracoscopy. Patients had a history of lung swelling in the past to give up surgery.
In September 2020, the patient was re -visited due to the aggravation of the symptoms, and the bronchoscopic biopsy was given: non -small cell carcinoma in the back of the left lung lobe, tendency adenocarcinoma.
Genetic testing: EGFR 21L858R mutation, the mutation is 14.1%. Tumor mutation load (TMB) is low, PD-L1 is positive.
Pathological: adenocarcinoma.
Previous history: history of mild qi.
Diagnosis: Multi -hair lymph nodes metastasis (phase III, T3N2M0), EGFR 21L858R mutation.
Provide experts
Professor Zhang Weihong: After EGFR-TKI resistance, MET amplification NSCLC brain metastase patients, Kizotininib to resist the efficacy of Savetinib
EGFR mutation is the most common type of mutation in patients with non -small cell lung cancer (NSCLC) in my country, existing about half of patients. At present, there are already many EGFR-TKIs in my country, which has brought hope of long-term survival to patients with EGFR mutations NSCLC. However, almost all targeted drugs will eventually appear to resist.
This case is a patient with multiple lymph nodes (phase IIIB, T3N2M0) with EGFR 21L858R mutations. The first -line treatment uses Ekitinib+left pulmonary radiotherapy. Monopodia+full skull radiotherapy second -line therapy. The effect is evaluated as a lung lesion SD, and the intracranial lesions PR. But 6 months later, the patient's PD, and the genetic testing prompts the resistance caused by MET amplification.
After that, the patient's treatment plan was converted into Oshitinib+Cizidinib, but the curative effect was not ideal. In 2 months, the chest CT was reviewed and the lung lesions were progressing again. Savininib is the first high-selective MET-TKI in my country that has been approved to be listed. Although there were previous studies and explored MET-TKI for a generation of EGFR-TKI drug resistance, MET amplification NSCLC, currently only Savacinib+Oshitinib dual target combination therapy is used for three generations of EGFR-TKI drug resistance after MET expansion Added research evidence support.
The TATTON research results show that among patients with EGFR mutations merging MET secondary and amplified NSCLC patients, Saverininib combined with EGFR mutations has considerable anti -tumor activity and tolerance. Treatment of patients, the objective relief rate (ORR) is 33%, and the median PFS is 5.5 months. The preliminary results of the recently announced Savanah research showed that dual -target therapy showed a promising clinical effect in the people of MET high amplification/high than expression (IHC 90+ and/or FISH 10+). The duration of duration (DOR) is 9.3 months, and the median PFS is 7.1 months.
Therefore, the patient was used for patients with Oshitinib+Saverininib for treatment. When the medication is 7 months, the curative effect evaluation shows that the lung lesions maintain SD, post -peritoneal lesion PR, and intracranial lesions PR; when the medication is 10 months, the efficacy evaluation is the overall PR. So far, patients have obtained PFS for more than 10 months and are still in benefits.
The treatment process of this case once again confirmed the long-term survival benefits of Oshitinib+Savidinib for EGFR-TKI drug resistance, and replaced Savo after treatment for Kizotinib for treatment for cizotinib. Tetini provides reference.
Expert Reviews
Professor Ren Xiubao: Successful "reversal" MET amplification resistance, EGFR-TKI combined with MET-TKI dual target treatment potential
MET amplification is an important mechanism for EGFR-TKI to obtain sexual resistance. Previous studies have shown that the incidence of MET amplification of first/second-generation EGFR-TKI in patients with sex resistance is 5%-20%[1,2]. In Aura3, the MET amplification ratio after the second -tier treatment of Oshitinib was 19%[3]. Flaura studies found that Osicinib first -line therapy did not have the acquisition resistance caused by T790M, and MET amplification (15%) was the most common obtaining drug resistance mechanism [4]. In addition, a review summarizes the molecular mechanism (tissue and/or plasma testing) of Osteinini resistance (tissue and/or plasma testing). %-50%, the incidence of MET amplification after the first-line treatment of Oshitinib was 7%-15%[5]. In the past, after EGFR-TKI was resistant to drug resistance, MET amplified NSCLC patients had poor treatment effects. With the listing of high-selective MET-TKI, it brought efficient and safe choices for such patients. Multiple studies have shown that the dual-target inhibitory effects of EGFR and MET channels can reverse the resistance to EGFR-TKI. Moreover, MET amplification NSCLC after three generations of EGFR-TKI is resistant to Divlc. At present, only Savininib and Oshitinib are supported by evidence.
TATTON research explores the efficacy and safety of the EGFR-TKI resistant NSCLC patients with Oshitininib dual-target for the treatment of MET. [6] The results showed that the first/second-generation EGFR-TKI patient patients with Oshitininib combined Saivininib treatment, regardless of whether the T790M is positive, shows good and lasting anti-tumor activity. In 11.1 months, Orr reached 62%-67%.
It is worth noting that the patients of TATTON study part of the B1 part of the three generations of EGFR-TKI were treated with the failure of the treatment of the treatment of the treatment. 96%of the patients were patients developing after the second and above. Accompanied by brain metastases. For this part of patients who are durable in the backline, the treatment of Savidi+Oshitinib can still bring 33%of ORR and the median PFS of 5.5 months.
At the just -held 2022 World Lung Cancer Conference (WCLC), Phase II SAVANNAH study announced the first data. Savatinib+Oshitinib joint treatment has progressed after receiving Oshitininib for the EGFR mutation and MET excessive expression and excessive expression and MET /Or MET enlargement local advanced or metastatic NSCLC patients add a new certificate. The results of the study showed that all patients received 32%of Oshitinini 80mg QD+Saverninib 300mg BID treatment, the median Dor was 8.3 months, and the median PFS was 5.3 months. Patients with higher METs are more significant for patients with Savinib+Oshitinib dual target. The ORR of IHC 90+ and/or FISH10+patients is 49%, the median DOR is 9.3 months, and the mid -bit PFS has also reached 7.1 months.
In the above cases, patients have been treated with the first and three generations of EGFR-TKI. After MET amplification resistance, first use Osidininib+Cizibininib therapy, but PD occurred 2 months later. After replacing Kizotinib to a high-selective MET-TKI Savidinib, the results of the efficacy evaluation in April 2022 showed that the patient's lung lesions maintained SD, postcard lesion PR, intracranial lesions PR; recently recently recently recently recently recently recently During a follow -up, the curative effect was evaluated as the overall PR. At present, PFS has reached more than 10 months, which fully demonstrates the excellent efficacy of Savacinib for MET to enlarge resistance. It is worth mentioning that most clinical experts currently use MET amplification copies to be greater than 5 as the CUTOFF value. Although the MET amplification multiplier is less than 5 times, this patient still has a good effect. In the future, the threshold of MET amplification will be Decision still needs to be further explored.
The efficacy of clinical research data and real world cases can be seen that MET-TKI has great potential for MET amplification resistance. In the future, with the continuous enrichment of evidence of evidence, MET-TKI is expected to benefit more patients with lung cancer.
Introduction to case review experts
Professor Ren Xiubao

Director of Biological Treatment of Tumor Hospital in Tianjin Cancer Hospital, Doctoral Tutor, Chief Physician, Doctor of Medicine
Director of the Biotechnology Research Office of the Institute of Tumor Prevention and Treatment of Tianjin
Deputy Chairman and Secretary -General of the China Pharmaceutical Biotechnology Association Medical Biotechnology Clinical Application Professional Committee
Executive Member of the China Anti -Cancer Association Cancer Biological Treatment Professional Committee
Director of the Tianjin Anti -Cancer Association
Director of the Sixth Council of the Chinese Institute of Immunization
Members of the 2nd China Human Genetic Resource Management Expert Group
Member of the Tianjin Anti -Cancer Association Lung Cancer Professional Committee
The Standing Committee Member of the Tianjin Anti -Cancer Association Cancer Rehabilitation and Passing Treatment Professional Committee
Member of the Tianjin Society of Biomedical Engineering Organization Engineering Professional Committee
Deputy Director of Tianjin Cancer Diagnosis and Treatment Center
Member of the Tumor Science Corporation of Tianjin Medical University
Introduction to experts
Professor Zhang Weihong

Department of Biological therapy, Tianjin Medical University Cancer Hospital
Deputy Chairman of the Tianjin Anti -Cancer Association Black Roman Professional Committee
Member of the China Medical Education Association of the China Medical and Biotechnology Association Clinical Applied Professional Committee
Member of the Tumor Professional Committee of Tianjin Medical and Health Society
Member of the Tianjin Anti -Cancer Association targeted therapy committee
American Danna-Farber Cancer Institute and
MOFFITT Cancer Center visiting scholars
references:
[1]. Pasquini G, GiaccoNe G. C-MET Inhibitors for Advanced Non-Small Cell LUNG CANCER [J]. Expert Opin Investig Drugs. 2018; 27 (4): 363-375.
[2].Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib[J]. Proc Natl Acad Sci U S A. 2007;104 (52): 20932-7.
[3] .papadimitrakopoulou v, et al. Analysis of Resistance Mechanisis to Osimrtinib in Patients with EGFR T790M Advanced NSCLC from the Aura3 Study.
[4].Ramalingam SS, Cheng Y, Zhou C, et al. Mechanisms of acquiredresistance to first-line osimertinib: preliminary data from the phase III FLAURA study. 2018 ESMO Congress. Abstract LBA50.
[5]. Leonetti A, Sharma S, Minari R, Et Al. Resistance Mechanisms to Osimertinib in EGFR-Mutated Non-Small Cell LUNG CANCER. [6].Lecia V Sequist, Ji-Youn Han, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a Multicentre, Open-Label, Phase 1B Study [J]. Lancet Oncol. 2020; 21 (3): 373-386. *This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform - END -

life imprisonment!Fire caused 46 people to die, 43 people were injured

On August 5, the suspect of the city in the city building in Kaohsiung City, Taiwa...
How can the earthquake cause secondary disasters, how to escape?
Source: Chongqing Earthquake Bureau