Metal's chemical practice
Author:Middle school chemical root ca Time:2022.08.28
High school chemistry synchronous lecture
Lecture: Teacher Zong Wei
Shaanxi Province's first provincial high school chemical backbone teachers and teaching experts, outstanding counselors of national chemistry competitions, senior chemical teachers in middle school, once taught in Xi'an No. 1 Middle School, Xi'an E -Science and Technology University Affiliated High School, West Institute of Technology Cultural Tuition School, and Xi'an Huanggang Cultural Tuition School
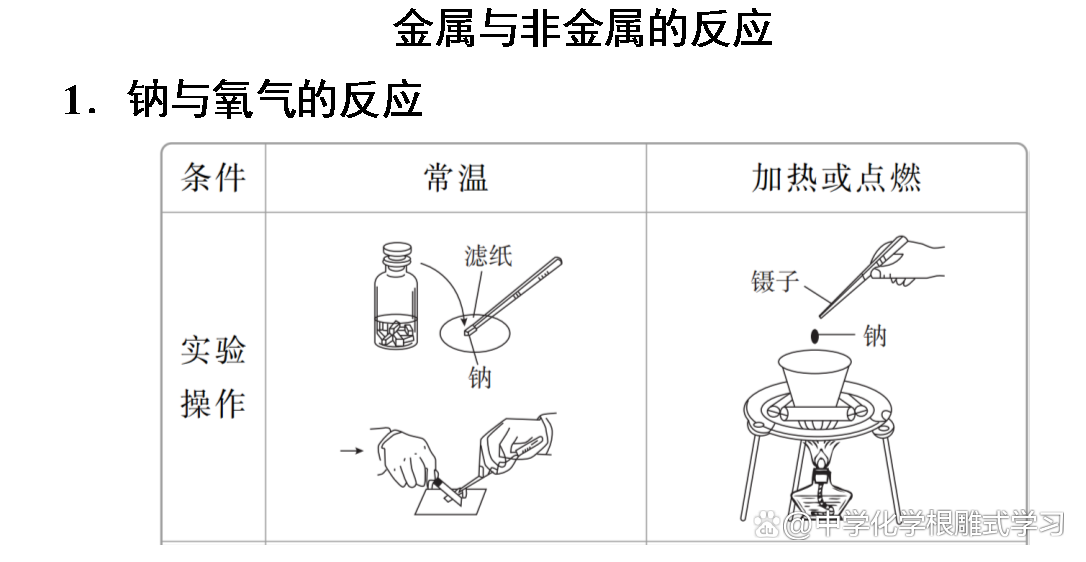

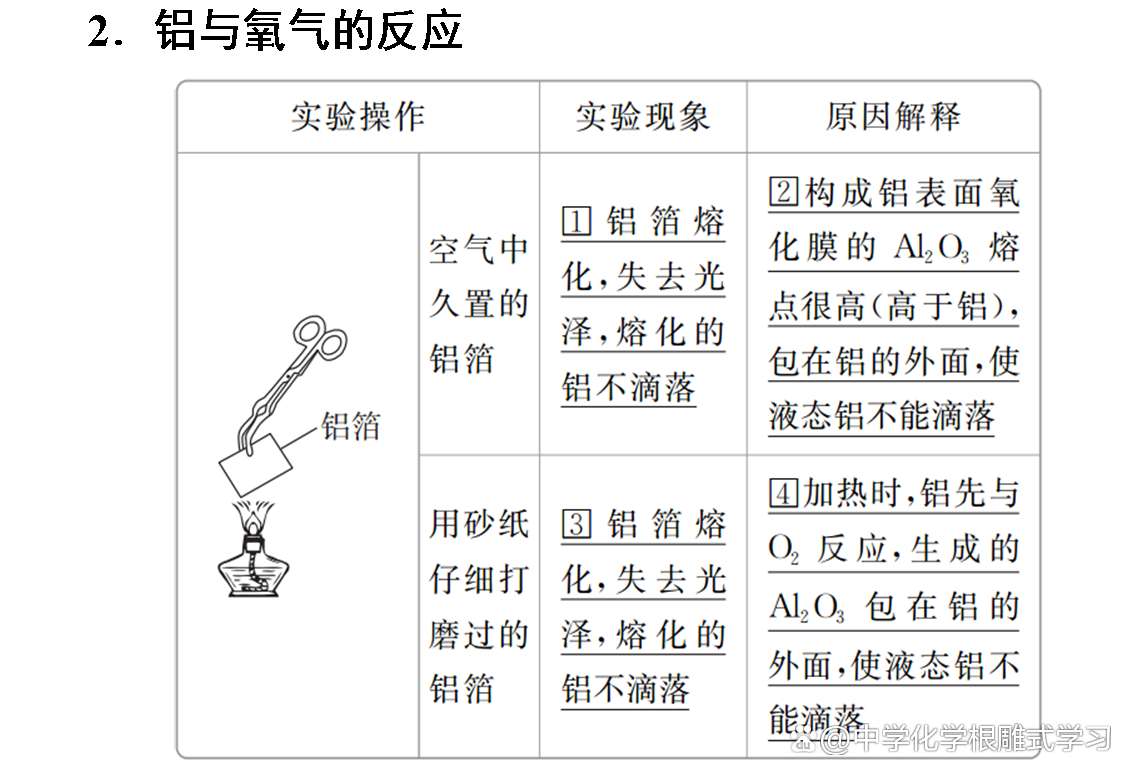
Sodium, iron, aluminum and other metals can be oxidized by oxidation in the air to form metal oxides. Sodium and iron can be completely oxidized, but aluminum products can be used for a long time in the air. Why?
The oxide film formed by aluminum is dense and can protect the inner metals from continuing to be oxidized. The oxide film of sodium and iron metal is loose and cannot protect the inner metals.
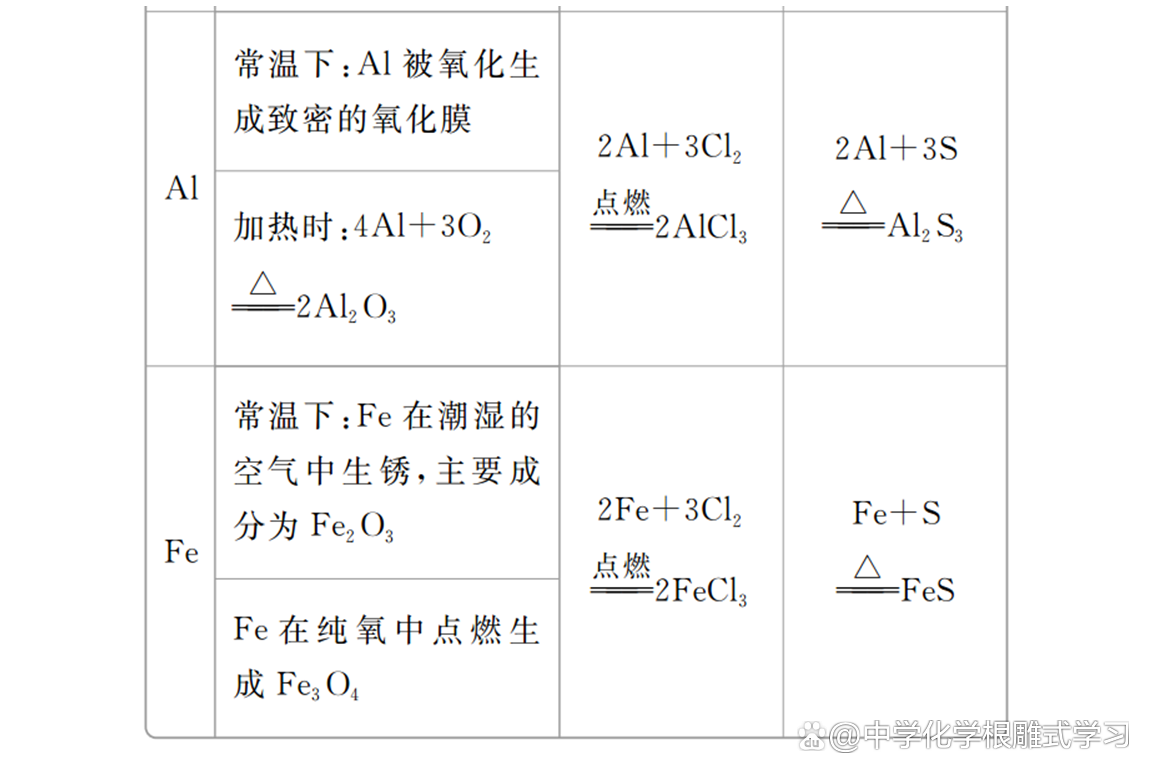

(1) Magnesium and aluminum have strong corrosion resistance; MG and AL chemical properties are lively, which is easily oxidized by oxidation in the air to form dense oxide film, which plays a protective effect on internal metals. , Frequent refractory materials.
(2) The price of iron and sulfur and chlorine reactions is different in the product. It proves that the oxidation of chlorine is stronger than sulfur.



- END -
Guojiapu Street, Dingcheng District: Repairing drought -resistant ports to ensure a bumper agricultural harvest

In the past few days, high temperatures have continued, and the Sanchahu Community...
Who do you say to blame this?

Today I will tell you a strong story.Dali and Xiaofeng played from childhood to bi...