Summary of chemical computing summary
Author:Middle school chemical root ca Time:2022.08.28
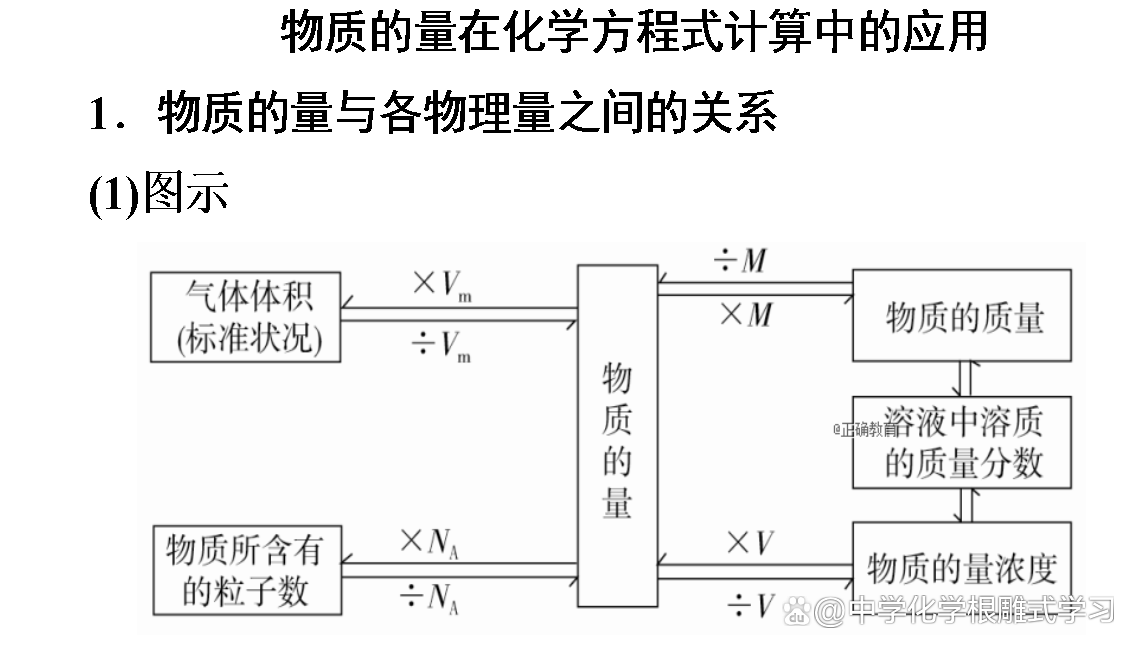
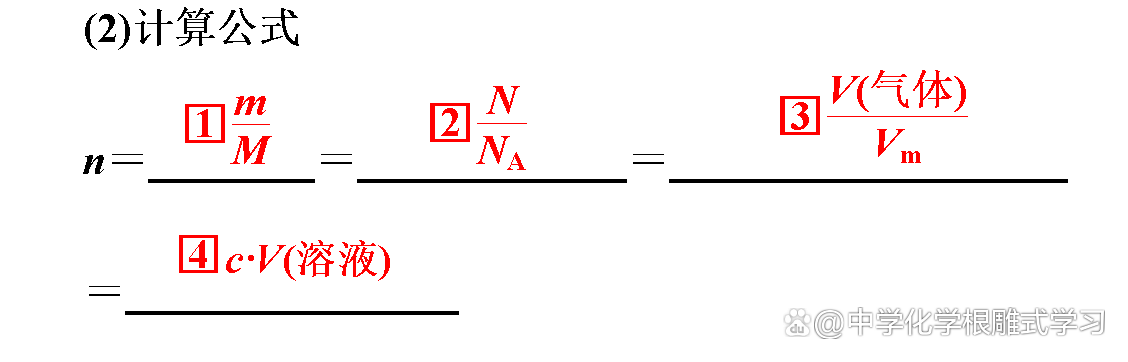
"The quality before and after chemical reactions is conservative, so the amount of material must be conservative." Is this right?
wrong. The ratio of the amount of substances in the substances in the chemical squares is equal to the ratio of the chemical measurement of each material.
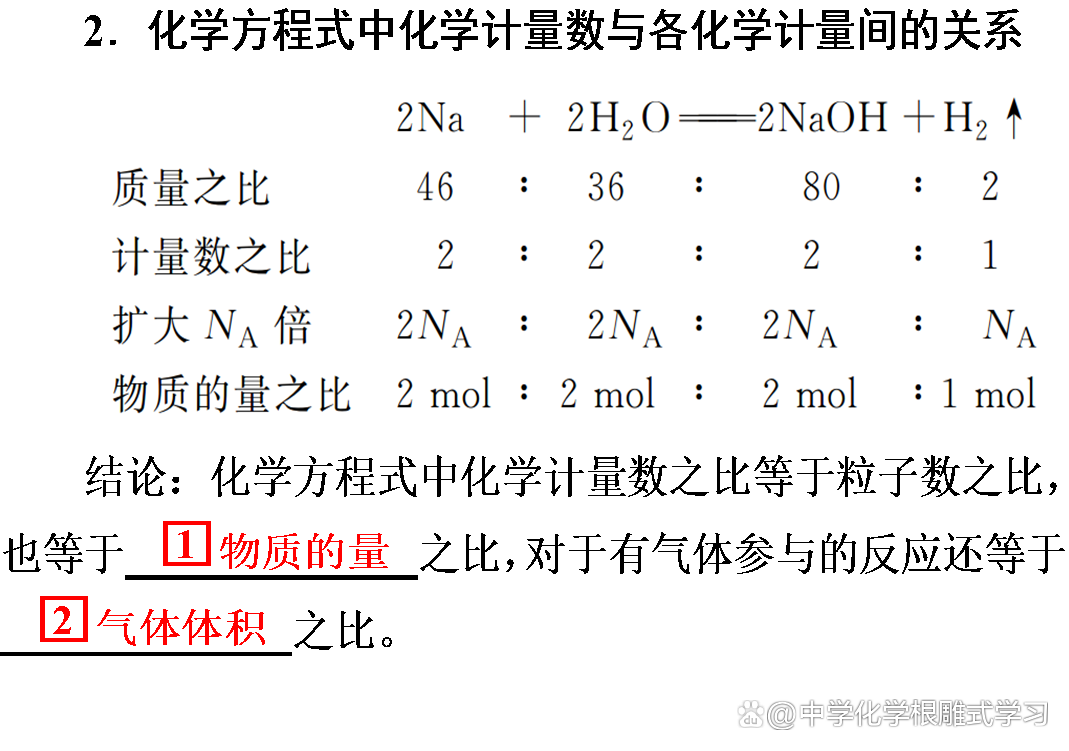
2. Two computing types
(1) Basic calculation
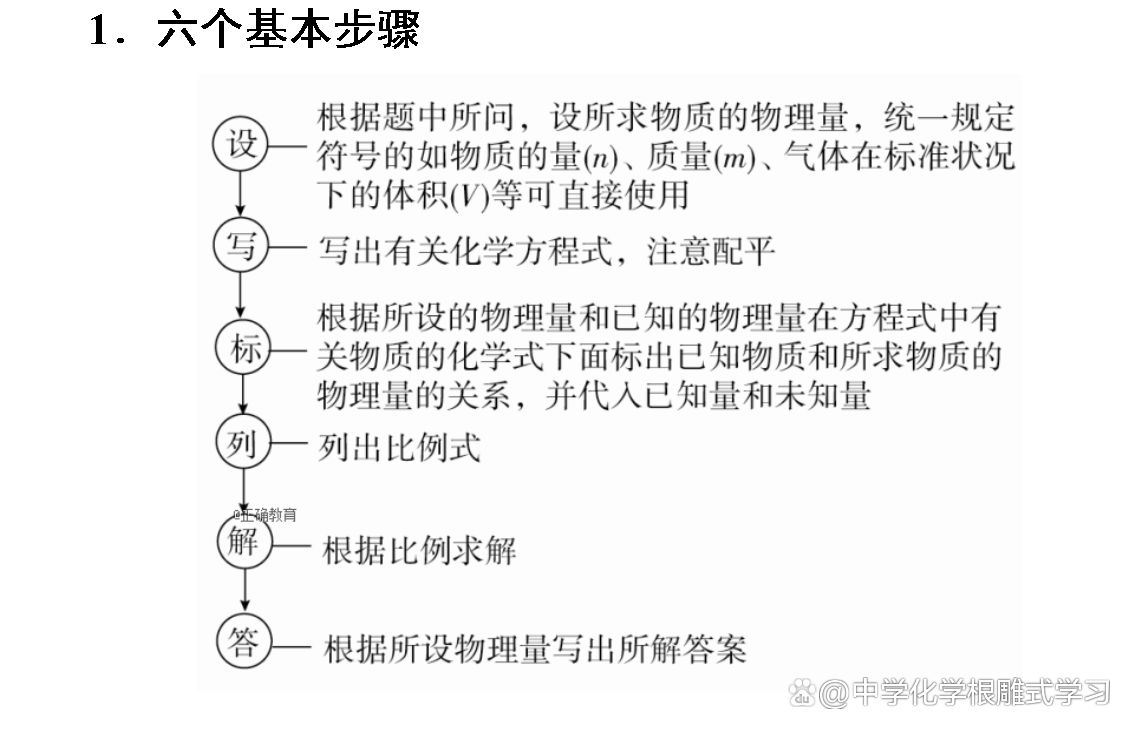
It is known that the amount of a reactor (or generic) solve the relevant amount of other substances. At this time, as long as it is calculated according to the relationship between the amount of chemical equations and the proportional formula of the known material and the proportion of material to be required.
(2) Excess calculation
The amount of two reactions is given to solve the amount of a certain product.
Method: Detect which substance is excessive according to the relationship between the amount of chemical equations, and then solve it according to the substances of insufficient amount.
(3) Calculation of mixture reactions
The amount of substances in the substances in the mixture is x, y, and z, according to the relationship between the amount of chemical equations, and expressed it with x, y, and z to listed the equation group to answer.
The 1.1 G iron and aluminum mixture are dissolved in 200 ml 5 mol·L -1 hydrochloric acid, and the concentration of hydrochloric acid after the reaction becomes 4.6 mol·L -1 (the solution volume change is ignored). beg:
(1) The amount of HCL consumed in the reaction.
(2) The amount of aluminum and iron in the mixture.

1. Two aluminums of equal quality react with sufficient amount of dilute sulfuric acid and sufficient NaOH solution, respectively.
A. 3: 2 B. 2: 3 C. 1: 1 D. 1: 2

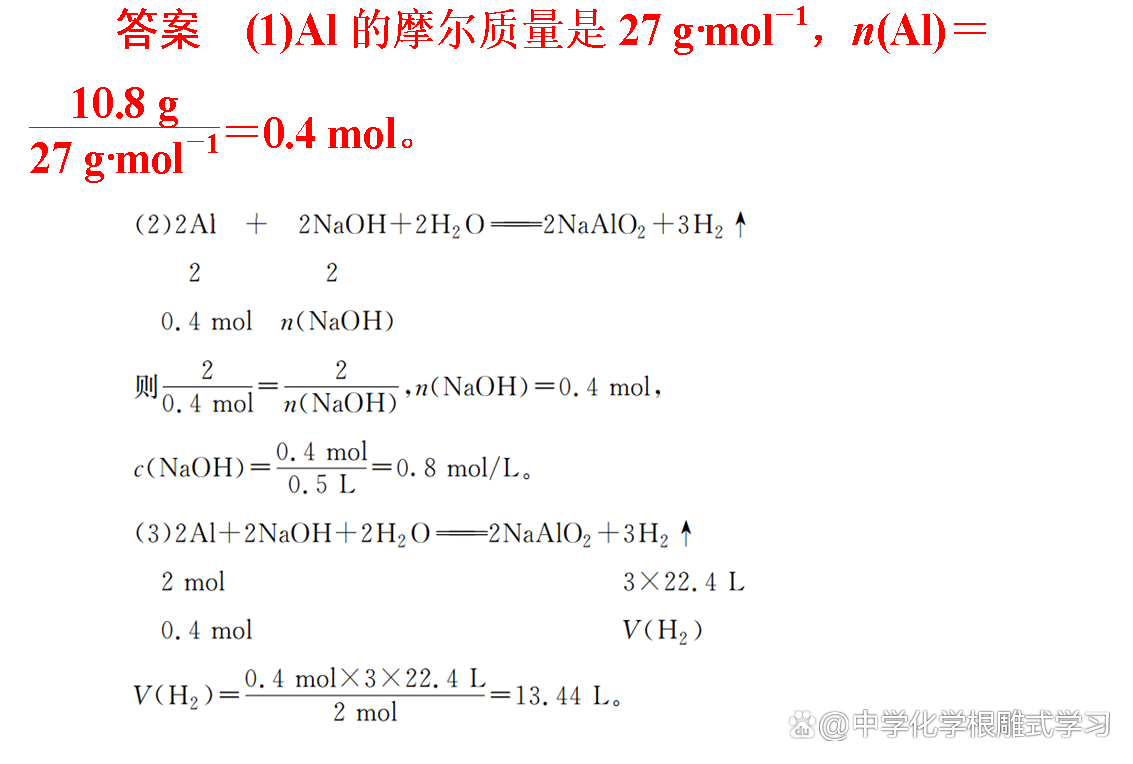
2. Invest in 10.8 g al in the 500 ml NaOH solution, the two just react completely, calculate:
(1) The amount of Al material.
(2) The amount of substances of NAOH and the amount of material of the material of NaOH.
(3) The volume of the generated H2 under standard conditions.
Put a sufficient amount of iron powder into a mixed solution of sulfuric acid and copper sulfate. After fully reacts, the quality of the remaining metal powder is equal to the quality of the original iron powder, then the amount of H + and SO4 (2-) in the original solution The ratio of concentration is ()
A.1: 4 B.2: 7
C.1: 2 D.3: 8

The method of judging excessive reactors
(1) Assumption method
假设一种反应物完全反应,而另一种反应物的量未知,把所求得的结果与实际量相比较,若小于实际值,则说明假设成立,反之则说明假设不成立,即该反应物excess.

In conclusion
1. Aluminum is a metal that can generate H2 with both acidic reactions and alkali solution reactions.
2. In the reaction 2AL + 2NAOH + 2H2O === 2NAALO2 + 3H2 ↑, aluminum is used as a reducing agent and water is used as oxidant.
3. The ratio of the chemical measurement ratio of each substance in chemical squares is equal to the amount of substances of each substance.
4. When calculated according to the chemical equation, the principle of the selection of physical quantities is: consistent up and down, equivalent to the left and right.
JPEG "Webkit-Playsinline>



- END -
The creation of the "Five Star" branch of Henan Qinyang made Qianxingfu Village a "Happy Village"

Recently, the breeding and breeding base of the meat rabbit breeding base in Xingf...
700,000 to 30,000 days savior "Xia Fan" Children with spinal muscle atrophy in Luzhou, Sichuan ushered in a new life
Huang Li Cover Journalist Jiang YuenRecently, the Affiliated Hospital of Southwest...