HER2 Positive Breast Cancer New Dawn: This domestic ADC "stands out"
Author:Cancer Channel of the Medical Time:2022.06.20
*For medical professionals for reading reference
The new hopes for the treatment of patients with late HER2 breast cancer are here: the objective relief rate is 65.5%, the disease control rate is 100%, and there is no progressive survival period for 17.02 months!
Recently, antibody coupling drugs (ADC) ushered in several heavy news in the field of breast cancer:
At the 2022ASCO Annual Meeting, DS-8201's Destiny-Breast04 studied as the only heavy studies selected as a general conference in the field of breast cancer and published it in NEJM at the same time. The crowd will expand from HER2 positive to HER2 low expression patients.
On June 7th, another domestic ADC drug Goshbuzabimab, which targeted Trop-2, was officially approved for listing to treat the non-removed local advanced or metastatic three-negative breasts that had been treated at least 2 systematic treatment in the past. Cancer patients.
On May 31, another ADC drug that targeted HER-2, which also paid high attention, was officially published in the top journal "Clinical Cancer Research), the ADC drug that targeted HER-2, the ADC drug of the ADC drug of the ADC drug. The results show that ARX788 shows excellent anti-tumor activity in HER-2-positive breast cancer patients and is safe and controllable.

Domestic new ADC: The result is encouraging
ARX788 is a new type of antibody puppetic drug. It consists of two parts: anti -HER2 monoclonal antibody and toxin small molecular AS269. Inhibitors PAF-AS269, inhibit cells with silk divisions, induced cell cycle stagnation and death.
This study is a phase I clinical study with a single center, openness, and dosage increasing dosage, which aims to evaluate the safety, tolerance and pharmacokinetic characteristics of ARX788 single medicine for HER2 -positive breast cancer.
The study started in 2017. A total of 69 cases of HER2 -positive breast cancer patients who have failed to treat HER2 in the past group. 82.6%of these patients received assistance/new assistive therapy, of which 75.4%had used ringling drugs, 72.5%of 72.5% After using patto drugs, 37.7%have used Tangzhu Mippital. After the metastasis, the average number of therapeutic lines of these patients reached 4 lines (2,14), 100%used Tushuzuke, and 7.2%of patients had used Puffyzumab. It is worth noting that 8.7%of patients have used other ADCs of Her-2.
The study set up 0.33 ~ 1.5mg/kgq3W and 0.88-1.3mg/kgq4W. As of the deadline for data, the study found:
①ARX788 Good safety: Among the 69 patients, no drug restricted toxicity (DLT) or death related to drugs occurred. Most patients (97.1%) have gone through at least one kind of adverse event (TRAE) related to treatment. Common (≥30%) TRAE includes elevated amino amino metastases, elevated amino aminotramerase, corneal Extraction of epithelial lesions, hair loss, hybrid hemophilia, interstitial pulmonary disease (ILD)/pneumonia and aldehyde solid ketone. Although 34.8%of the subjects occurred in quality pneumonia, only 2 were severity level 3.
②Arx788 is considerable efficacy: under 1.5 mg/kg Q3W (Recommended dose in Phase II), the objective relief rate (ORR) is 65.5%(95%CI, 45.7 ~ 82.1), and the disease control rate (DCR) is 100%(95%CI). , 81.2 ~ 100), the median without progress (PFS) is 17.02 months (95%CI, 10.09 ~ not reached).
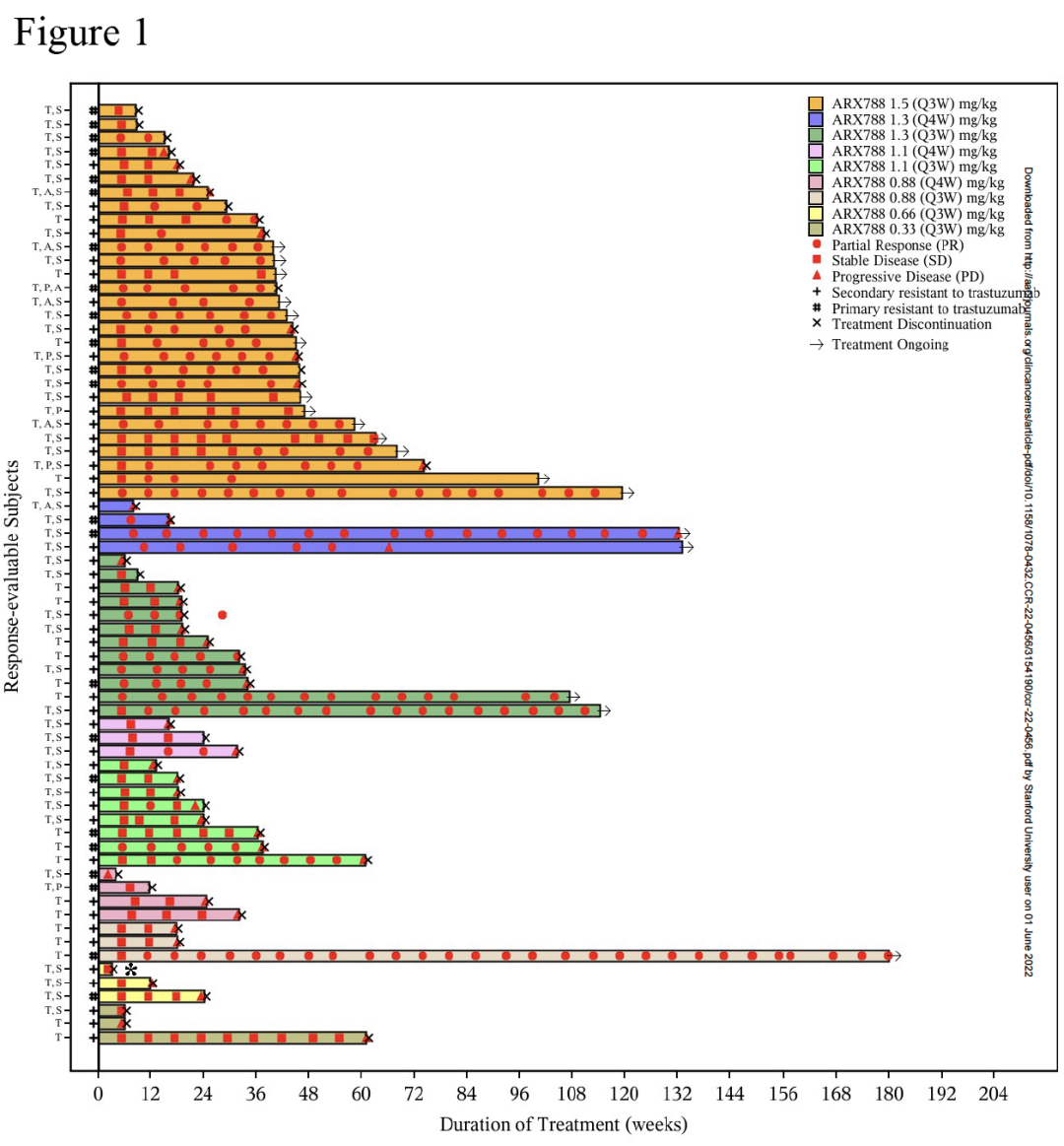
Figure: Survival Trail Picture
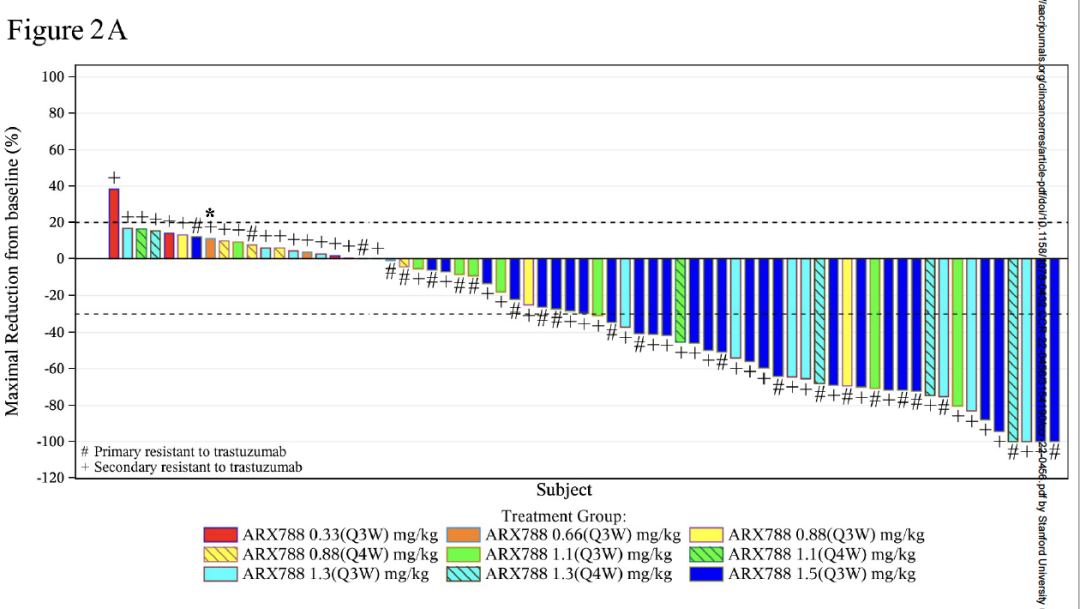
Figure: Waterfall map
Efficacy Excessive expectations: It is expected to become a new optional medicine
After the article was published, the "Medical Tumor Channel" contacted Professor Zhang Jian, the first author of the article. He said: "ARX788 has received everyone's attention for a long time. It is said that the entire R & D track is in a relatively advanced position. This medicine has a significant effect in the field of HER-2-positive gastric cancer, and has obtained the qualification of FDA orphan drugs. "
Compared with other advanced breast cancer treatment drugs including ADC, ARX788 has the following characteristics:
① Unique drug structure design: The connection between this drug's anti -HER2 monoclonal antibody and toxin small molecule AS269 is special. It belongs to non -natural amino acids (other ADCs are mostly natural amino acids). The ratio of the drug antibody ratio (DAR) reaches a fixed proportion 2, so compared to other ADCs, there are fewer AS269 that falls off to the plasma (only 1.1%of small molecules released in the blood), and the systemic toxicity is relatively controllable.
② The efficacy exceeds expectations: In Phase I, ORR reaches 65.5%, and the disease control rate is 100%. In particular, PFS can reach 17 months. Compared with the ADC of ADC, which is currently listed in my country, it has a significant advantage. Essence
③ Safety is generally controllable: Compared to other ADCs, no systemic toxicity such as hematology, gastrointestinal tract, and gastrointestinal tract is not observed compared to other ADCs. Oncology hospitals have also set up a series of management processes for interstitial pulmonary disease, strictly monitoring suspected patients, and timely adjusting the dose of drugs. The overall level 4 toxicity or death has not occurred. ④ No obvious cross -drug resistance with other ADCs is not observed: 4 cases (80%) of patients who have used anti -HER2 ADC have been objectively relieved in the Asian group analysis. It may still be valid to use another ADC, because the carrons between different ADCs are different from the connectors, and the technical platform is different. This also provides more ideas for our domestic clinical treatment and new drug research and development.
"In general, ARX788 is a relatively good anti -HER2 ADC drug, which may become a new choice for the treatment of HER2 positive breast cancer in my country in the future. At present, Professor Hu Xichun team is leading the stage of phase II clinical research. At the same time, ARX788 is also conducting the treatment of patients with HER-2 low-expression breast cancer and HER-2 brain metastases. At present, a certain effect is observed. I look forward to the formal research results in the future. Add.
references:
[1] zhang j, ji d, shenw, xiao Q, gu, o'Shaight J, Hu X, Phase I trial of a Nivel Antibody-Drug Conjugate, ARX788, for theTreatments Cancer, Clinical Cancer Research, May 2022, Doi: 10.1158/1078-0432.CCR-22-0456
Expert Introduction
Professor Zhang Jian

The Administrative Director of the Phase I Clinical Research Ward of the Cancer Hospital of Fudan University, Chief Physician
Shanghai "Medical Garden Star" Jieqing talent winner
Standing Committee Member of the Breast Cancer Professional Committee of China Anti -Cancer Association
Vice convener of the Youth Committee of the Breast Cancer Professional Committee of the China Anti -Cancer Association
Yangtze Academic Band Breast Alliance YBCSG Chairman
Deputy Chairman of the Youth Committee of the Breast Professional Committee of China Research Hospital Association
National Vice Chairman of the National Anti -Cancer Drug Clinical Application Monitoring
Breast cancer integration and prevention and control of the National Expert Committee Vice Chairman of the Youth Committee of the National Expert Committee
The chairman of the Shanghai Anti -Cancer Association Cancer Drug Clinical Research Committee
Deputy Chairman of the Shanghai Anti -Cancer Association Cancer Pharmaceutical Committee
CSCO tumor support and rehabilitation treatment expert committee
CSCO Youth Expert Committee Standing Committee Member
CSCO Breast Cancer Expert Committee member
The first batch of clinical part -time reviewers of the State Food and Drug Administration CDE
Deputy editor of "Diseases & Research"
"Translational Breast Cancer Research" and "Gland Surgery" editorial board
First/Common First/Communication Author published more than 40 SCI papers ("Lancet Oncol", etc.)
The first release of this article: the medical world tumor channel
Author of this article: Yu Xiaosu
Editor in charge: Sweet
- END -
Mom's simple wish
□ Chen LanMom has five golden flowers. From the beginning of the sensation, I know that my mother has a wish that has not been achieved: having a son, the end of the elderly for her elderly, and bl
Luchuan County Meteorological Bureau lifted lightning yellow warning signal [III level/heavier]
The Luchuan County Meteorological Bureau lifted the thunderbolt yellow warning signal at 09:03 on June 08, 2022.