The first domestic new crown and antibody drugs on sale, nearly 10,000 yuan a course of treatment, is expected to prevent Omikon?
Author:First financial Time:2022.07.08
08.07.2022
Number of this text: 2072, reading time for about 3 minutes
Guide: When the US FDA evaluates whether a neutral antibody is valid, the evaluation index should be the concentration of neutralized antibodies in the lungs, not the concentration of the blood, and the result may be up to 20 times.
Author | First Financial Money Children's Heart
On July 8th, at the press conference of China's commercialization listing conference in China, the new crowns and antibodies of the new crowns and antibodies in Tengshengbo Medicine, as the first self -developed new crown virus in China In the combined treatment of drugs, Tengsheng Bo drugs revealed the information of the drug pricing. The domestic price of 2 grams of dose is not higher than 10,000 yuan.
During the treatment, this pair of neutralized antibodies is mainly through intravenous injection. The two medicines are injected with 1g each. The whole process can be completed in less than an hour.
As of the close of the day, Tengshengbo's stock price rose by more than 11%. The distributors of Anbawei's Mipidum and Romi Siweizab include Chinese Medicine Holdings, Shanghai Medicine Group, China Resources and so on. In addition, the company revealed that it is discussing the drug as a national strategic reserve with relevant departments.
Responsible for Omikon's dispute over the escape
On December 8 last year, Anbawei's combination therapy was approved by the State Drug Administration (NMPA) listing for the treatment of light and ordinary types (including hospitalization or death in the treatment of light and ordinary types (including hospitalization or death. ) A new type of coronary virus infection with high-risk factors (12-17 years old, weight ≥40 kg).
For the prevention effect of neutralized antibodies that are highly concerned about the new mutant strains of Omikon, Tengsheng Pharmaceuticals announced data that the Ambaleab and Lemisville's anti -combined therapy neutralized Omikon BA. The activity of 4/5 is similar to BA.2. The pharmacokinetic model shows that after a week of injection, the blood concentration IC50 (50%virus inhibitory rate) is greater than 100 times, and the IC90 (90%virus suppression rate) is close to 20 times , Explain that it is effective for Omikon BA.4/5.
According to Teng Shengbo Pharmaceuticals, according to the National Institute of Health (NIH)/National Institute of Allergic and Infectious Diseases (NIAID), 837 cases of Activ-2 studied clinical trials of 837 patients with outpatient patients show Compared with the comfort agent, the long -term Ambalab and Romi Siwei's anti -combination therapy reduces the hospitalization and death risk of new crown outpatient patients with high risk of clinical progress by 80%, which is statistically significant. As of the clinical end of the 28 days, the treatment group was zero death and the placebo group had 9 deaths, and its clinical safety was better than the placebo group. At the same time, patients who have started treatment (within 5 days after the symptoms appear) or patients who begin to receive treatment (within 6 to 10 days after the symptoms appear), the hospitalization and mortality rate will be significantly reduced, which provides longer patients with new crown patients with longer patients with a longer crown patient. Treatment window period.
However, before the data of Professor He Dadi, a virus expert at the University of Columbia, the antibodies including Ambarab and Romi Siwei's anti -combination therapy will be escaped by Omikon's mutation to a certain extent. Last month, Professor Xie Xiaoliang's team of Peking University and Changping Laboratory published research papers in the "Nature" magazine also showed that the combined therapy of Anbavir Midumi and Romi Siwi could not be effective Neutralization.
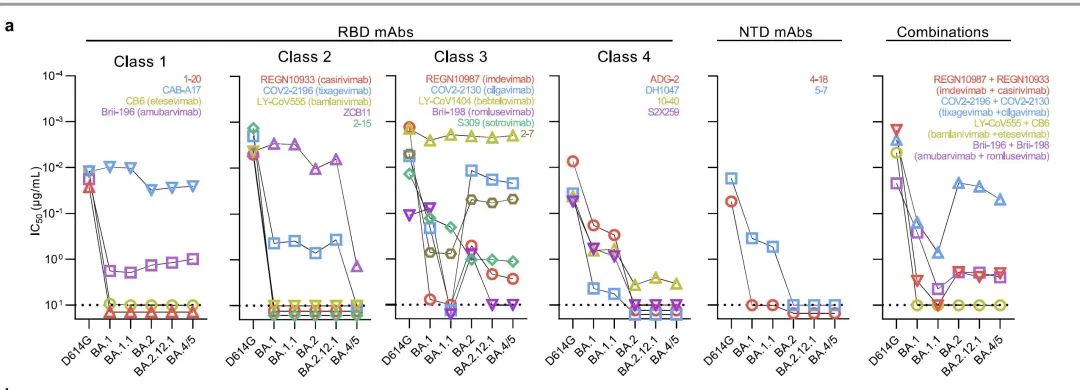
In response, Dr. Zhu Qing, senior vice president of Tengshengbo Pharmaceutical and head of the biopharmaceutical department, said: "The neutral test in the laboratory of Professor He Dayi is in vitro testing. The test results of each laboratory are different. Although the combination of Antimaru and Romi Siwei's anti -combination therapy for Omikon's activity has decreased, it still retains part of the activity. "
Teng Shengbo also said that in addition to the pharmacokinetic model, increasing dosage can also enhance the efficacy of antiviral virus. The company's executives introduced at the press conference that even if the drug was given 3 grams, the data proved to be safe and tolerant. "Even if the new crown has new mutations, our antibodies can still be effectively respond."
Pre -exposure Prevention Introduction has the prospects
The combination of Antimetabu and Romi Siwei's anti -combination therapy currently approved in China is the treatment of the new crown. Essence "This combined antibody therapy was originally taken into account the use of prevention when the antibody was designed, and the use of genetic engineering reconstruction technology was used to prolong the half -life of long -acting antibodies." Teng Shengbo Pharmaceutical executives said.
Some industry experts have pointed out that because the Omikon mutant strains have reduced the cost of many neutralized antibodies, the dose of antibodies must be increased.
"Medication is likely to lead to more mutations in the evolution of viruses in the condition of insufficient efficacy but not increasing dosage." A relevant field experts told the first financial reporter.
Another expert also stated that it is worth noting that when the US FDA evaluates whether a neutralized antibody is valid, it examines the pharmacokinetics of neutralized antibody drugs in the lungs, rather than the peripheral blood published by Teng Shengbo medicine. Dynamics. Since the concentration of antibody drugs in the lungs is far less than that of peripheral blood, the result may be more than 20-50 times the difference, so the effectiveness of Abavir Mipide and Romi Savidab on BA.4/BA.5 is effective on BA.4/BA.5 How to observe sex.
At present, Teng Shengbo has launched clinical research that the antibody is used for pre -exposed prevention, but the company has not yet provided the effective data of this drug to prevent new pre -exposed infection. Zhu Qing said: "From a scientific perspective, this drug can greatly reduce the rate of infection, but whether it can completely block the infection still needs to be further studied." Recently, the world's only one that is used for pre -exposure of the new crowns to prevent prevention prevention. The neutral and antibody Evusheld (Enbishi) developed by the drug, developed by Astraon Company has completed the approval of special items in the country and arrived in Hainan. The one -time two -pin vaccination price was 13,300 yuan.
Regarding Evusheld and Anbawei's combination of anti -anti -anti -anti -anti -anti -alive therapy may compete in the Chinese and global markets in the future. compete."
Effective new crown drugs are of great significance to control the popularity. Professor Lu Hongzhou, Dean of the Third People's Hospital of Shenzhen, told the first financial reporter: "At present, there are local outbreaks of BA.2 and BA.5 mutant strains in my country. Wei Shan has a strong antiviral effect, and compared to antiviral small molecular drugs, neutralizes antibodies also have a long clinical treatment time window. There is no need to consider the advantages of patients with liver and kidney function and drug interaction. "
- END -
"Why don't you get married?"

Seeing this title, is there any tightness?Don't worry, we don't urge marriage.01Re...
Focus on the Provincial Party Congress | Creative empowerment, so that the Internet Red Scenic Area will go further

Visitors experience the net red glass water slide in the Red Leaf Stock Rock Sceni...