Nuggets Innovation Pharmaceuticals | The first domestic dual -resistant "card point" was approved by the Kangfang Biology to get medical insurance negotiation coupons?
Author:Daily Economic News Time:2022.07.08
"Nuggets Innovation Pharmaceuticals" was jointly launched by the Daily Economic News and Data Data. It aims to interpret the progress and trend of new drug research and development, analyze product competitiveness and market prospects, insight into pharmaceutical capital context, and witness the high -quality development of the pharmaceutical industry.
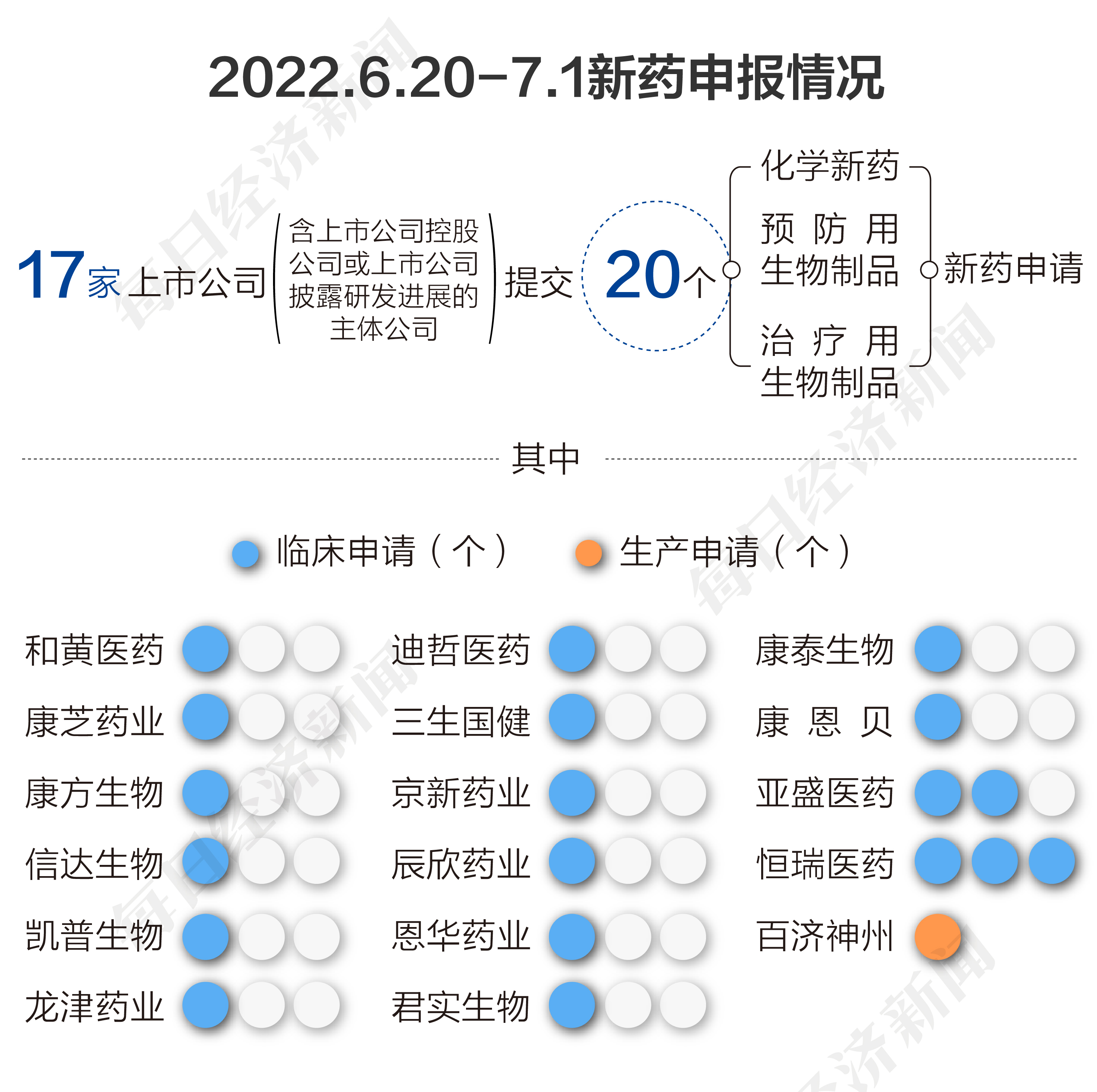
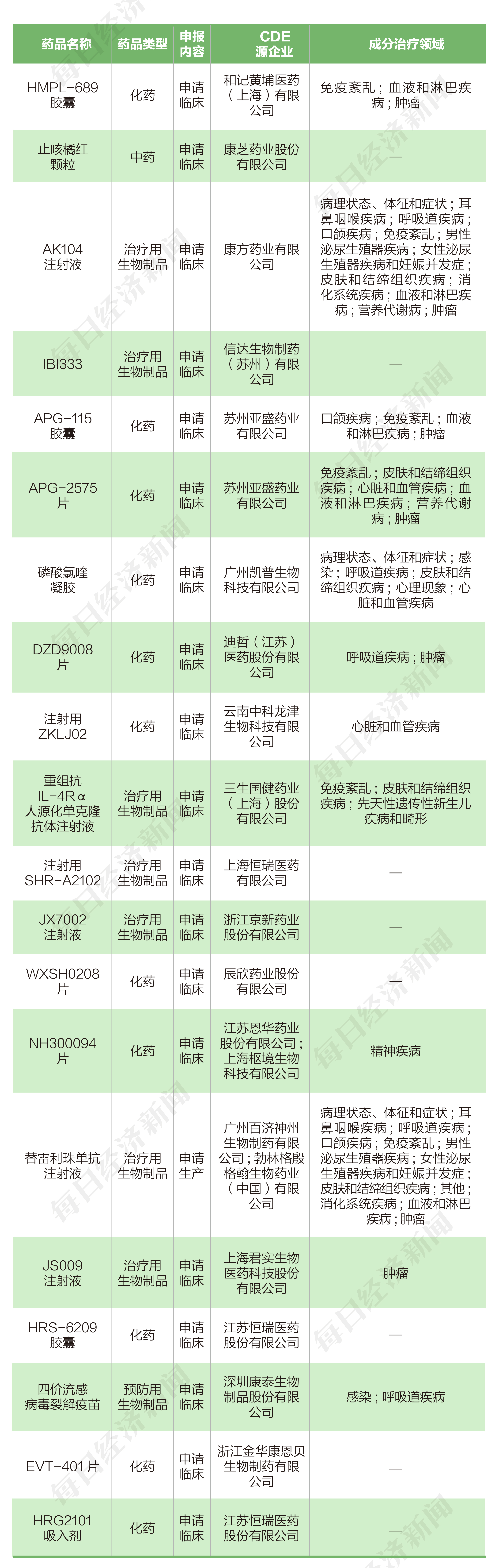
According to the pharmaceutical data, from June 20, 2022 to July 1, 2022, a total of 17 listed companies (including listed companies or listed companies disclosed research and development progress from the National Drug Administration of Drug Administration (CDE) The main company of the main company) submitted 20 chemical new drugs, prevention of biological products, and new drug applications for the treatment of biological products.

New drug application
From June 20th to July 1st, 2022, aspects of listed companies, Hehuang Medicine (00013.HK), Kangzhi Pharmaceutical (300086.SZ), Kangfang Bio (09926.HK), Cinda Bio (01801 .Hk), Kaipu Bio (300639.SZ), Longjin Pharmaceutical (002750.SZ), Dazhe Pharmaceutical (688192.SH), San Life Guojian (688336.SH), Jingxin Pharmaceutical (002020.sz ), Chenxin Pharmaceutical (603367.SH), Enhua Pharmaceutical (002262.SZ), Junshi Biological (688180.SH), Kangtai Bioscope (300601.SZ), and Kang Enbei (600572.SH) were submitted by 1 A clinical application. Yasheng Pharmaceutical (06855.HK) submits two clinical applications, and Hengrui Pharmaceutical (600276.SH) submits three clinical applications. Baiji Shenzhou (688235.SH) submits a production application.
New medicine hot review
1. Can the first domestic double resistance approve the average annual cost of 200,000 yuan to make the Kangfang creature "turn"?
On June 29th, Kangfang Bio -Cardonabitab Injection (AK104, Commodity Name Kaitini) approved the listing through priority review and approval procedures, suitable for recurrence or metastatic cervix that was previously received in the failure of platinum chemotherapy treatment. Treatment of cancer patients. Kadinili is the first domestic double resistance to the listing, and it is also the world's first PD-1/CTLA-4 dual resistance.
Kadinilicabeta is a two-specific antibody that targets PD-1 and CTLA-4, which can block PD-1 and CTLA-4 and its ligand PD-L1/PD-L2 and B7.1/ The interaction of B7.2, thereby blocking the immunosuppressive response of PD-1 and CTLA-4 signaling pathways, promoting tumor-specific T cell immune activation, and then giving play to anti-tumor effects.
The approval of the Kadinilicab is based on the results of a Phase II key clinical study carried out in China. Patients in the test group were patients with recurrence or metastatic cervical cancer who had previously received platinum chemotherapy. The results of the study were published at the Annual Meeting of the American Gynecological Oncology Society (SGO) in 2022.
At present, Kadinilicab is also developing a variety of malignant tumors including non -small cell lung cancer, liver cancer, gastric cancer, cervical cancer, kidney cancer and nasopharyngeal cancer. A variety of studies are in phase II or stage III phase. Among them, the progress of metastatic esophageal cancer and metastatic gastric cancer is relatively fast.
Industry insight:
From the perspective of the domestic cervical cancer treatment market, cervical cancer is one of the most common gynecological tumors in the world and my country. Among female malignant tumors, its incidence is second only to breast cancer. Data show that 604,000 newly issued worldwide in 2020, 342,000 deaths, corresponding to 109,000 new issues in my country and 59,000 deaths.
In the field of cervical cancer prevention and treatment, cervical cancer vaccines have previously popularized, but for patients with relapse or metastatic cervical cancer, the need for treatment is also urgent. In addition, compared with early cervical cancer, the prognosis of recurrence or metastatic cervical cancer is limited.
According to the first edition of the "NCCN Cervical Cancer Clinical Practice Guide" in 2022, cisplatin is considered to be the most effective drug for metastatic cervical cancer. First of all, Pablizumu Microex+Cisplatin (or Card Platinum)+Pucinol ± Bearing bead monocycles (level 1 evidence).
For patients who cannot undergo surgery or radiotherapy, cisplatin, card platinum, or paclitaxel are a reasonable first -line single -medicine solution. Among them, cisplatin is the most effective chemotherapy single medicine for recurrence or metastatic cervical cancer. For patients with chemotherapy for cervical cancer during the same period, cisplatin single drugs are usually preferred. Those who are not tolerated by cervical platinum are also considered to use card platinum. The guide for cervical cancer's second -line therapy drugs mainly include albumin paclitaxel, Pam Mibu, Dorcio, and Bedarzumab.
This means that the main competitors of Kadinilicab will be Parbryzumab (K medicine), Bervarzumab, Nawuli Mippitive (O drug) and many biological similar medicines. The first few major research drugs have been listed in China for many years, and have expanded a number of indications. According to relevant data, the sales of Bevarzumab in the first quarter of 2022 reached 2 billion yuan, Paborizumab reached 400 million yuan, and Nawuli Ulitab reached more than 90 million yuan. At the same time as the market prospects are broad, Cunnili Metarius will also sing many "seniors".
In addition to the competition with the original research medicine, there are already many biological similar drugs in China in China. In 2019, Angda, Qilu Pharmaceutical, was approved as the first imitation, and sales reached 1.8 billion yuan in 2020. After Qilu Pharmaceutical, there were several Bayzab's similar drugs in China.
Company comment: On June 27th, 29th, and 30th before and after the approval of Kadinili, the stock price of Kangfang fell 2.55%, 3.95%, and 5.14%respectively; The stock price rose 14.75%. The ups and downs of the stock price may represent the outside world's view of the approval of the Kangfang biological dual anti -drugs to a certain extent.
Image source: Wind screenshot
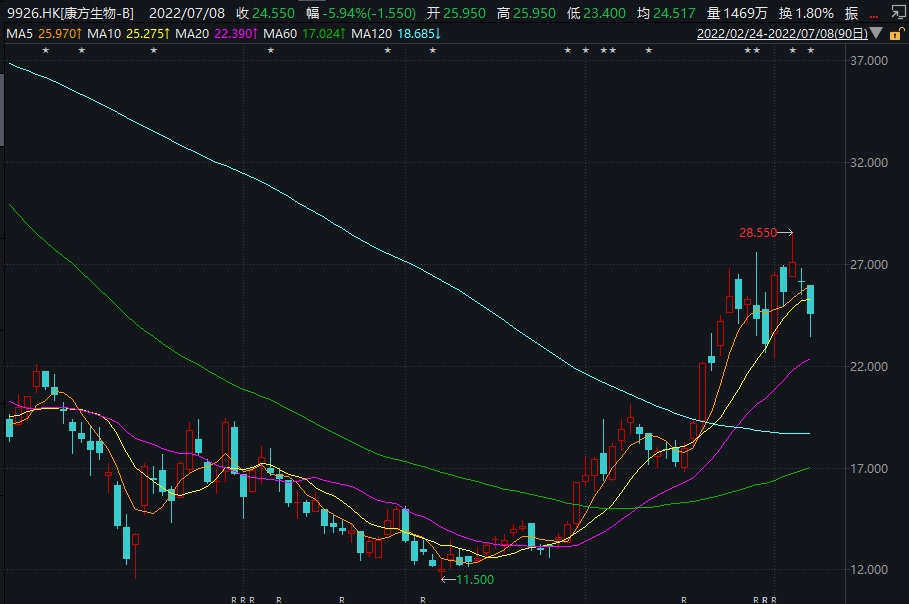
As the biggest highlight of the Kangfang creature or the biggest expectation of the outside world, the approval of Kadinilicab is a new milestone for the company, and it also verifies its own research and development strength. However, as mentioned earlier, the market performance after the approval of Kadinili was related to whether the closed loop of the Kangfang creature from R & D to commercial profits could be opened.
Xia Yu, the founder and chairman of Kangfang Bio and Chairman, said in an interview with "Nuggets Innovation Pharmaceuticals" in 2021 that the commercialization of Kadinilicab will be carried out by the company and revealed that the company is already setting up its own commercial team. Essence The annual report of Kangfang Biological 2021 shows that the company's sales team has exceeded 500 people and plans to expand to 800 in 2022.
However, Kangfang Bio has no experience in the commercialization of new drugs independently.
In 2021, its first commercial product PD-1 was sent to be listed for sale. The way of Kangfang Bio adopted was to combine Zhengda Tianqing to promote the commercialization of this drug. The product was listed for 4 months, and the sales were sent to the sales of about 212 million yuan, which performed in a lot of PD-1 products. But during the same period, the sales cost of Kangfang Biological was not low -reaching 179 million yuan. In addition, due to the late listing time, it was sent to the opportunity of medical insurance negotiations last year.
After Kadinili Meticulum is on the market, the outside world is most concerned about its pricing problem.
In early July, the price of Kadinili monoclonal resistance was released -13220 yuan/125mg/bottle, with 3 bottles each time, and administered every two weeks. According to the pricing plan and the patient rescue plan, the annual treatment cost is not higher than 198,000 yuan. However, it is beneficial to the more Aplitabi resistance that according to the approval time of the drugs set up this year's medical insurance negotiations, the Kadinili Monopoctomy "stepped on the point" to catch up with this year's medical insurance negotiations.
"Nuggets Innovation Pharmaceuticals" researcher believes that in the future, if Kadinili Mipido can be successfully included in the medical insurance directory through negotiations, it will be expected to accelerate the speed of entering the hospital through medical insurance to achieve the acceleration volume of the product. Whether it will be observed whether to apply for the adjustment of the medical insurance directory and the quotation of the medical insurance catalog and the quotation of the medical insurance directory this year.
2. Oukang Vision's treatment of non -infectious uveitis is approved to fill in domestic gaps
On June 20, the official website of NMPA showed that OT-401 (fluorine easily vitreous implanted, Yutiq) was approved by CDE new drugs to treat chronic non-infectious uveitis that accumulated the rear. This is the first new drug in the history of Chinese medicine registration to be fully declared based on "real world research data".
OT-401 (Yutiq) was introduced by Okang Vision in November 2018 from Eyepoint Pharmaceuticals. It is the first of similar drugs, non-biodegradable micro-mitigation injection-type internal agents. The active ingredient of OT-401 (Yutiq)-fluorine, fluorine, is an artificial synthetic corticosteroid. It has been widely used for 30 years as local anti-inflammatory drugs. Within 3 years of implantation of the outpatient clinic, a total of 0.18 mg active ingredients are continuously released at the preset control speed.
OT-401 (YUTIQ) was first approved by the US FDA on October 18, 2018, and it is by far the only drug to treat uveitis. On November 30, 2020, OT-401 started the "real world research" in Boao, Hainan. In December of that year, OT-401 was officially included in the list of the first batch of drug pilot species of the drug and device clinical world data of the Hainan Provincial Drug Administration.
From the perspective of the real-world data mid-term report, the use of OT-401 can significantly reduce the recurrence rate of uveositis after Pattern patients with NIU-PS, and gradually increased vision after implantation. The amount of potion decreases significantly. Compared with traditional treatment methods, the recurrence rate of OT-401 sets of uveitis, a significant vision increase, and a significant decrease in hormone usage.
Industry insight:
Esmitis is a kind of eye inflammation and one of the main causes of blindness around the world. If it is not treated, blindness will be the natural course of this disease. Sugitic hormone and immunosuppressive agents are traditional drugs for the treatment of gluciditis. Although they can temporarily prevent the development of the disease, long -term use has obvious toxic and side effects.
Not infectious uveitis is a chronic uveitis, which can cause various complications such as cataracts, glaucoma, vitreous turbidity, macular lesions. As a result, permanent vision is lost, so it is also the second most blind eye disease in China.
According to the Fifrrishalvan report, the number of patients with chronic non-infectious uveitis in China in 2022 is about 1.5 million patients. The patient group is about 45,000 to 75,000.
OT-401 is the first FIRST IN CLASS drug that can continue to stabilize in the eyes to release fluoride in the eyes. The characteristics of the continuous action time have been approved to fill the gaps in the field of uveitis. Therefore, in the domestic market, OT-401 is still a competitive pattern. Company comment:
In addition to OT-401, it has been approved to be listed. Up to now, Oukang Vision has 20 drug assets in front of the eyes and back to the back of the eyes, of which 6 products have entered the phase III clinical trial. The company's pipeline realizes the full coverage of myopia, dry eye and bottom disease.
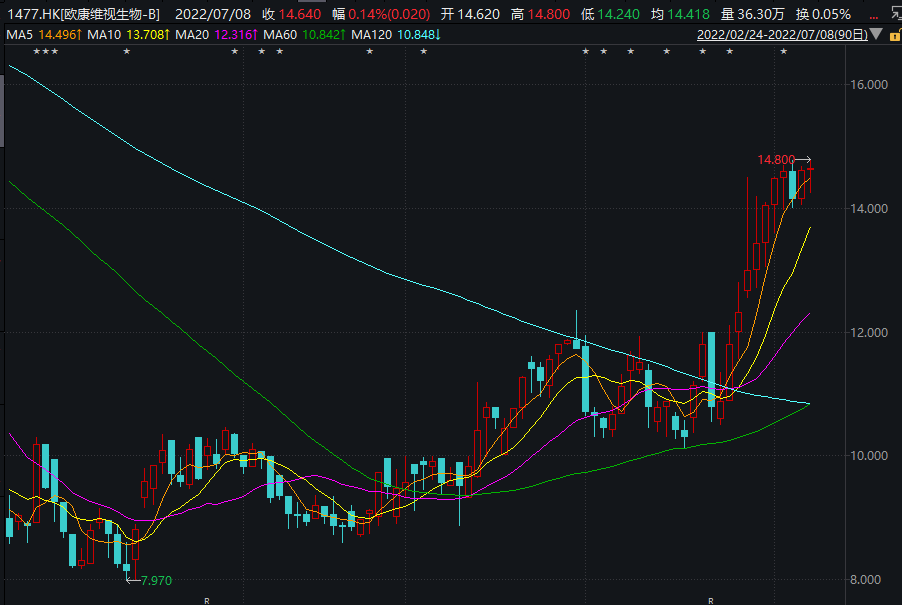
In 2021, due to the increase in sales revenue of dry eye and glaucoma products, the company's revenue increased from RMB 113 million to 56 million yuan, and the company's net loss narrowed from 277 million yuan in 2020 to 187 million yuan in 2021. After the announcement of OT-401, Oukang Wei's stock price rose significantly.
Image source: Wind screenshot
In the pipeline of Oukang Vision, there is also a layout for adolescent myopia control drugs. Unlike Xingqi Eye Medicine (SZ300573, the stock price of 107.22 yuan, 9.4 billion yuan) and other R & D companies. The III phase III International Multi-Center Clinical Test (MRCT), the direction of commercialization is registered and listed in many countries around the world; the second is OT-101 launched a real world research in Boao, Hainan last October; the third is that OT-101 uses an exclusive design of innovative and confined types Skin device to solve the problem of stability of low -concentration atropine solution.
At present, there is no domestic eye drops in China. Facing the broad market of teenagers, many companies are watching.
However, the researcher of "Nuggets Innovation Pharmaceutical" noticed that there have been rumors in the industry that relevant departments have begun to rectify Atropine's eye drops. Without approval, they have sold in the form of Internet hospitals and in -hospital preparations in advance. Xingqi Eye Medicine and other representatives of corporate stock prices have fallen shock on multiple trading days. If the clinical trials are progressing smoothly, companies that have advanced to the clinical stage of clinical III are expected to take the lead in the formal market of Atropine's eye drops.
Daily Economic News

- END -
Focus on supporting the strong economy and strong economy with technology.
The Yangtze River Daily Da Wuhan Client June 25 (Reporter Wu Yan Chang Shaohua) On June 26 (Sunday) at 15:00, the Yangtze River Daily will launch an online interview program of Cloud Living Room. In
Tangshan Nanhu lit "Midsummer Night" as a real text travel to benefit the people

I was looking forward to it very much before, the plot of Fanghua That Year was ve...