The results of the world's first PD-1 inhibitors head-up research results were announced: Civili Single Single Anti-Domestic Immunohic Drug Ling Cancer First Line Treatment "Strongest Voice"
Author:Cancer Channel of the Medical Time:2022.07.14
*For medical professionals for reading reference
CTONG1901 [1] Study, the world's first forward-looking head-to-head comparison comparison between two PD-1 inhibitors (Xindiley Mipide VS Paborzab), the efficacy and safety of the domestic immune drugs for lung cancer The "strongest voice" of treatment!
The treatment plan for PD-1/PD-L1 inhibitors/combined chemotherapy has now become the standard choice for the first-line treatment of non-small cell lung cancer (NSCLC) in the drive gene. Many PD-1/PD-L1 inhibitors independently developed by my country, their performance in clinical practice is no longer inferior to foreign imports of drugs. However, as of now, there are no clinical research adopting a "head-to-head" forward-looking design, which directly compares the efficacy of different immunotherapy drugs in lung cancer. Domestic PD-1/PD-L1 inhibitors and similar imported drugs. What's more?
At the just end of the 2022 ASCO Annual Conference, Ctong1901, led by Professor Wu Yilong, the People's Hospital of Guangdong Province (Abstract No.: ABSTRACT #9032), for the first time directly compared the original Chinese research PD-1 inhibitors Xin Dili Mipida and foreign research and development abroad. PD-1 inhibitors Paborzab's curative effect and safety in the first-line treatment of late NSCLC.

The "Medical Tumor Channel" specially invited Professor Ma Zhiyong, Henan Cancer Hospital to comment on the results and significance of the CTONG1901 research.
Excellent efficacy brings confidence, CTONG1901 studies "qi" for China's original research immunotherapy drugs
CTONG1901 Study is a open label, random, and phase II clinical study. It was initiated by the Chinese chest tumor collaboration group (CTONG) led by Professor Wu Yilong. The research head opposite compared the efficacy and safety of the Syrumab and Paborzab in the late NSCLC first -line therapy. Professor Ma Zhiyong pointed out that "there were few two PD-1 inhibitors directly comparing the study. Most PD-1 inhibitors were compared with simple chemotherapy."
In terms of research design, PD-L1 expression level was used as a group factor, and patients were divided into 4 groups. Among them, patients with PD-L1 TPS ≥50%, randomly accept the treatment of Xinyi Midumi (group A) or Paborzab (group B) single drug treatment; and PD-L1 TPS <50%of patients are randomly accepted by Xinyi Licipopea+chemotherapy (group C) or Paborzumab+chemotherapy (group D) combined treatment. The main end of the study is Orr (recist 1.1).
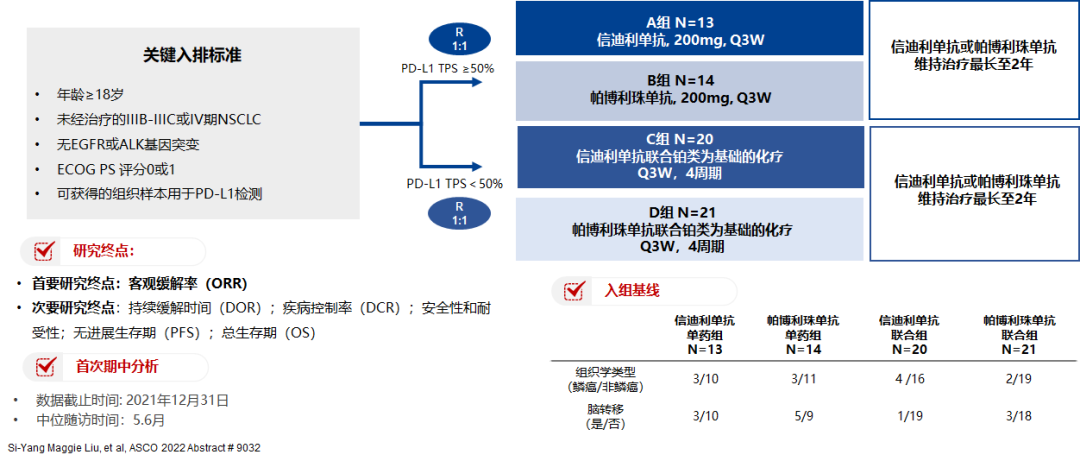
Figure 1.Ctong1901 Study the overall design
Summary of the treatment and combined therapy of single drugs, the orr of the Sini Mipido group ORR reached 51.6%(16/31), and the Paborizumu monetary group was 30.3%(10/33). As a stage II study with a small number of sample samples, the CTONG1901 research can not be concluded that two types of PD-1 inhibitors are good or inferior, but Professor Ma Zhiyong said, "It is certain that it is, Xinyi Lishan Single Single Single Single Single Single Shan Regardless of whether it is a single medicine or combined chemotherapy, there is no difference between the efficacy and Paborizumab, and this is the clinical clinical clinical of the first-line treatment and safety of the first two PD-1 inhibitors in the country and the world. Studies. I want to initiate this study, which shows that my country's clinical researchers and pharmaceutical companies are full of confidence in independently developed immunotherapy drugs. The research results also confirmed that their curative effect can be completely compared with imported drugs abroad. "
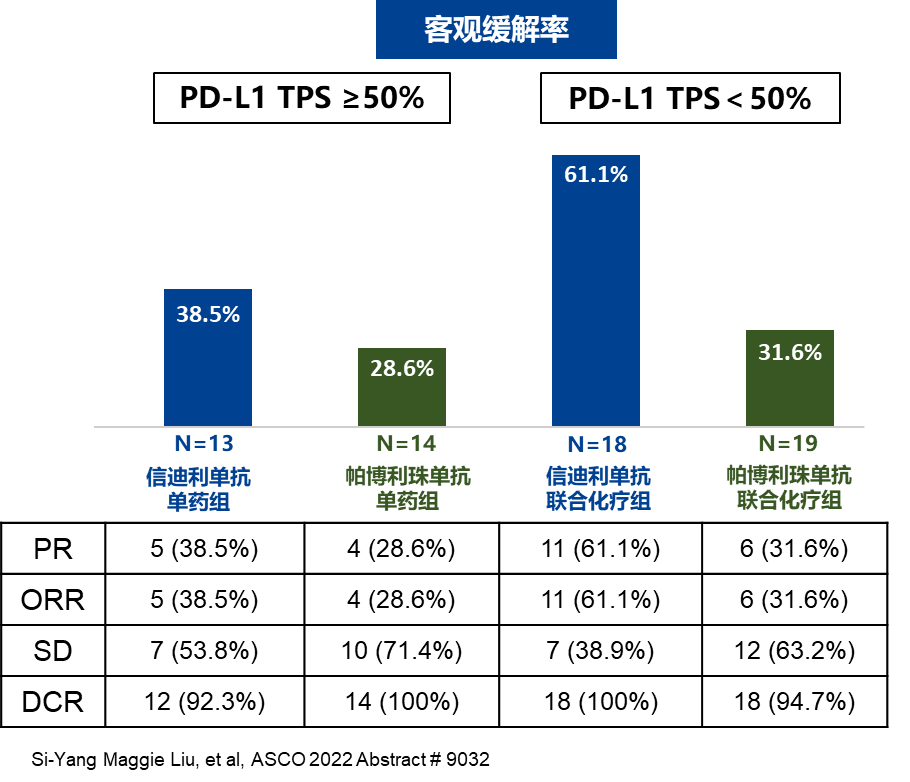
Figure 2. The relief rate and disease control rate of patients in each group
Professor Ma Zhiyong believes that the success of the CTONG1901 research not only further verifies the international quality of Xinyi Mipida and its status in the treatment of advanced lung cancer, and enhances clinic workers' confidence in its efficacy and safety. From a higher level of perspective, it will also bring confidence to the international market for independent innovation drugs in my country. Based on the Chinese patients, Xinyi Metico has a clear treatment advantage
The indicator of the treatment of lung cancer treatment in China in my country is based on its Orient series research. At present, the Orient-11/12 Study of the first-line treatment [2-3], and Orient-31 [4] (EGFR- A number of large -scale III clinical studies such as TKI resistance) have been successful. Orient series studies are included in Chinese patients, which has better guiding value for clinical practice of clinical practice.
In Professor Ma Zhiyong's view, "the clinical research of Xindili Monopoly is very solid, providing good evidence -based medical evidence, and in the subsequent real world, you can also see the Xindi Lili counterfeit The incidence of adverse reactions is low, no special adverse reactions occur, safe and controllable, which has been commonly recognized in clinical practice. "
Based on Orient-11/12 research, Xindi Lilitab has been approved for local advanced or transferred non-scale NSCLC and scale NSCLC. Medical insurance directory.
Professor Ma Zhiyong pointed out that Xindi Lilitab was included in medical insurance, allowing more patients to use international first -class immunotherapy drugs at a more friendly price, which significantly reduced the economic burden of patients and increased the possibility of long -term use of immunotherapy. Especially with the improvement of the overall therapeutic effect of NSCLC, more and more patients who have long -term treatment and long -term survival are increasing, reducing the burden of these patients cannot be underestimated. Shinyi Meticuke can provide reliable choices for these patients.
Based on the present and looking forward to the future, Xindi Lili continues to be full of sailing
The dazzling performance of the CTONG1901 research at the ASCO Annual Meeting is just one of the many clinical studies of Xindi Mu Mu. Professor Ma Zhiyong believes that the long-term efficacy data such as the non-progressive survival period (PFS) and the total survival (OS) of the Orient-11/12 research have fully displayed the status and value of Xindi Mipida in the late NSCLC first-line therapy. The next step can continue to pay attention to NMPAs of the study of Orient-31 and other studies.
Based on the successful clinical research of the advanced NSCLC, Xindi Lilitab is also expected to continue to march to the NSCLC new assist/auxiliary therapy and the treatment of small cell lung cancer to achieve full coverage in the field of lung cancer treatment. international market.
Expert Introduction
Ma Zhiyong

Henan Cancer Hospital
Chief physician, master student teacher
Director of Henan Provincial Lung Cancer Diagnosis and Treatment Center
Director of the China Clinical Oncology Society (CSCO)
Standing Committee Member of the China Anti -Cancer Association Lung Cancer Professional Committee
CSCO Non -small Cell Expert Committee Standing Committee
Standing Committee Member of the China Medical Promotion Cancer Specialist Committee
Chairman of the Professional Committee of the Lung Cancer Professional Committee of the Henan Provincial Anti -Cancer Association
references:
[1]Liu S Y M, Zhou Q, Yan H H, et al. Sintilimab versus pembrolizumab in monotherapy or combination with chemotherapy as first-line therapy for advanced non–small cell lung cancer: Results from phase 2, randomized clinical trial (CTONG1901)[ J]. Journal of clinical onCology, 2022, 40 (SUPPL 16): 9032.
[2]Yang Y, Wang Z, Fang J, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double-blind, phase 3 study (Oncology pRogram by Innovent Anti-PD-1-11) [J]. Journal of Thoracic Oncology, 2020, 15 (10): 1636-1646.
J]. Journal of Thoracic Oncology, 2021, 16 (9): 1501-1511. [4] s. Lu*, L. Wu, H. Jian, et al. Orient-31: Phase Study of Sintilimab With or WithoutIBI305 Plus Chemotherapy in Patients with EGFR Mutated Nonsquamous NSCLC Who Progressed after EgFR-TKI Therapy [J] .2021 ESMO Asia Virtual Plenary .2021 ESMO Asia Virtual Plenary.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
The Ministry of Public Security launched the "Hundred Days Action" to promote the "Hundred Days of Action" to promote the development of normalization and elimination of evil struggle
Poster reporter Liu Lu Beijing reportOn July 15, the Ministry of Public Security held a press conference to report the 100 -day action of summer public security to combat the 100 -day action. Jia
Cao County, Shandong: The lotus in the summer solstice is blooming

On June 21, tourists watched lotus (drone photos) in the National Wetland Park of ...