The first -line chemotherapy of gastric cancer has progressed rapidly, and OS exceeds 2 years after second -line treatment!
Author:Cancer Channel of the Medical Time:2022.07.16
*For medical professionals for reading reference
Pickings and learn wonderful "miracles" case!
Gastric liver-like adenocarcinoma (HAS) is a rare special type of gastric cancer, which refers to a gastric cancer that is originally characterized by gastric mucosa and has the characteristics of differential differentiation of adenocarcinoma and liver cell cancer. The incidence is about 0.17 % -15 -15 %, More common in elderly men, mostly occur in gastric sinus and gastric body. It is prone to liver metastasis and lymphatic metastasis, and high invasion. AFP positive gastric cancer patients have a poor prognosis. As AFP rises, the 5 -year total survival (OS) rate decreases.
In this issue, the "Classification of Cases" was shared by Professor Huang Ming, a professor of the Cancer Hospital affiliated to Fudan University.
Basic situation of patients with advanced gastric liver -like adenocarcinoma
The 66 -year -old male patient was visited in April 2020 due to "abdominal fullness and discomfort".
On April 30, 2020, gastroscopy shows: hyperthyroidism gastrointestinal glandular cancer, Lauren classification: mixed type. Her-2 ++, FISH negative. The abdomen CT shows that the stomach wall is thickened, the lymph nodes are metastasis around the stomach, and the liver is more metastasis.
Past history: no history of hypertension, diabetes.
Family history: None.
Physical examination: ECOG1 points, superficial lymph nodes are not swollen, abdomen is soft, mobility is voiced (-), and both lower limbs have no edema.
Tumor marks: baseline AFP3080ng/ml.
From April 2020 to August 2020, the 5 -cycle Osarina Platinum + Dajio Plan Chemotherapy, the best effect is: stable disease (SD). AFP gradually increased. On August 3, 2020, AFP rose to 10430 ng/ml. In August 2020, the liver metastasis progressed compared to forward, and disease progress (PD).
In August 2020, he went to the Cancer Hospital of Fudan University. The pathology will be diagnosed with immunohistoclation: AFP+, SALL4+, HEP-PAR 1-, consider hepatic adenocarcinoma, PMMR.
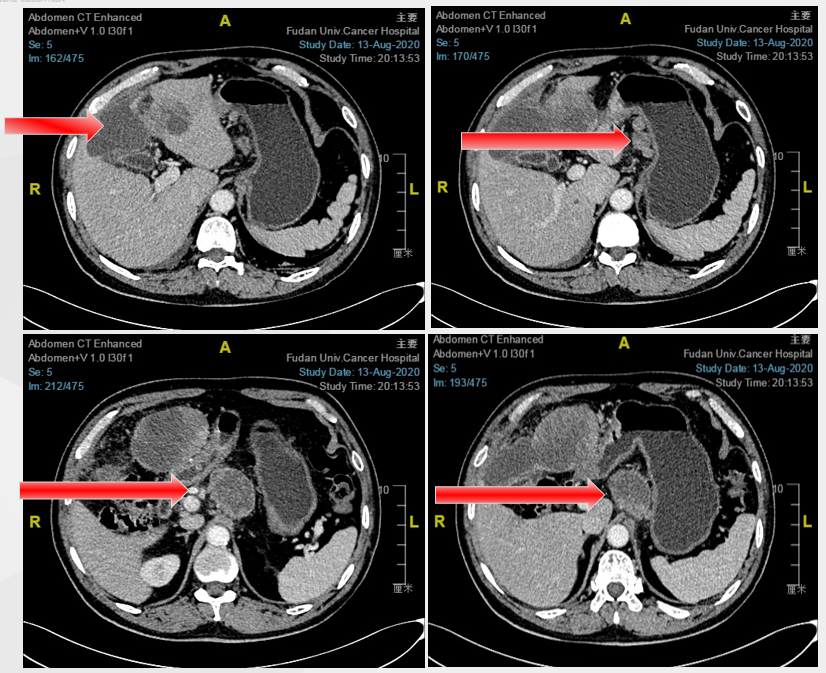
August 2020 baseline imaging examination
ask
Why choose Apatinib combined with PD-1 monoclonal anti-anti-anti-treatment?
Considering that the patient's previous Osarina, the treatment effect of the treatment of Jiogo, the non -progressive survival period (PFS) is only 3 months, and IHC prompts gastric liver -like adenocarcinoma. Therefore, it is recommended that patients consider participating in the comparison Clinical study of placebo therapy. Because the clinical study needs to be cleaned for 2 weeks, patients and family members do not consider clinical research in the group.
The second -line standard treatment of advanced gastric cancer is the treatment of paclitaxel or Illi -Kangpan drug, and patients still have concerns about chemotherapy. Then refer to the treatment of liver cell carcinoma (HCC), combined with the treatment of gastric adenocarcinoma, try Apatinib+PD-1 monoclonal anti-anti-anti-treatment.
HAS still has no standard treatment plan, and only about 13%at the time of diagnosis is early. HAS lacks thymosine phosphatase, but is rich in dihydrial hydrogenidase (DPD) may be one of the reasons for the difference in routine chemotherapy schemes.
AHEAD-G202 Studies are an open, forward-looking, multi-center clinical research. This study aims to evaluate the efficacy and safety of ant vascular genera Apatinib in the advanced stage of nail protein (AFPGC) Essence Patients undergo oral administration of Apatinib single drug treatment or received joint treatment. The study evaluated the efficacy of 20 patients receiving Apatinib for patients. The objective relief rate (ORR) of patients with the Apatinib group was 10 %, and the disease control rate (DCR) was 70 %. The median PFS is 3.5 months (95 % CI: 2.34-4.66), and the median OS is 4.5 months (95 % CI: 3.49–5.51).
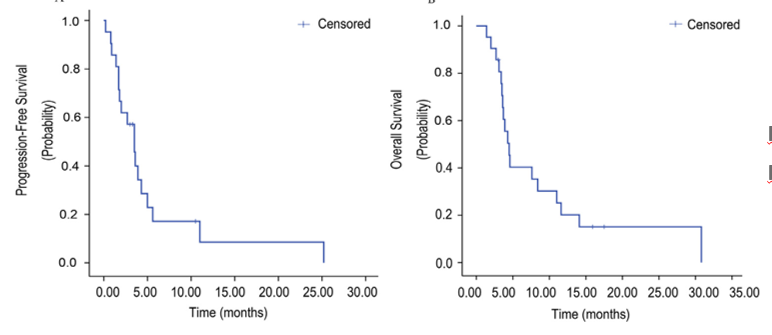
AHEAD-G202 studies the median and medium OS of patients with the Apatinib group
The median OS of AFPGC patients with an increased carcinoma antigen (CEA) reached 30.8 months. Through the multi -variable analysis, the CEA elevation is considered to be the potential independent predictive factors of OS (P = 0.030) and PFS (P = 0.047).
A total of 21 patients with advanced chemotherapy, a combined chemotherapy treatment of Professor Liu Tianshu's team affiliated to Fudan University. Of the 21 patients, seven patients accepted the PD-1 antibody (Nawuli Yuab) immunotherapy and chemotherapy, and 14 patients who were in contrast received a combination or non-combined Togkukeab/Apetinib Chemotherapy. The results of the study showed that the median PFS of the control group and the immunotherapy group was 4.3 months VS 22 months (P = 0.01), and the median OS was 16 months and did not reach (P = 0.03).
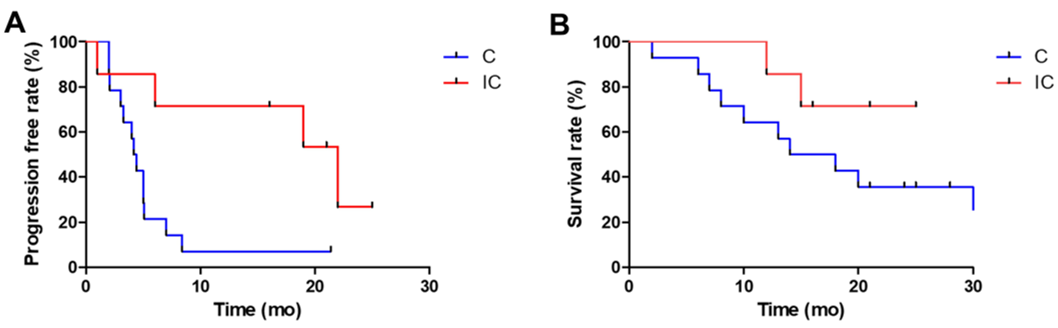
Professor Liu Tianshu's team carried out the median PFS and median OS of the control group and immunotherapy group
Immune combined with anti -vascular production treatment,
PFS over 20 months
August 20, 2020
The patient began Apatinib combined with PD-1 monoclonal anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-treatment treatment. During the treatment period, the Ⅰ degree of white blood cells decreased, and the degree I was weak. After 4 weeks of treatment, AFP dropped to 5380ng/ml, and the efficacy assessment was SD.
From August 2020 to the present
The patient continued to receive Apatinib+PD-1 monoclonal anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-anti-treatment treatment. As the treatment time is prolonged, the number of white blood cells decreases, the Ⅰ degree of thyroid function decreases, and Ⅰ degree RCCEP. The efficacy assessment is the continuous part of the relief (PR), and AFP continues to decrease.
April 13, 2021
The serum AFP is 26.7 ng/ml.On April 29, 2021, the abdomen enhanced CT of the Cancer Hospital affiliated to Fudan University: The stomach wall thickened the same front, the lymph nodes in the stomach and the liver gate area were reduced, and there were multiple nodules in the liver.On July 2, 2021, the serum AFP was reviewed by 12.2 ng/ml. On July 5, 2021, the abdomen CT showed that the stomach wall was thickened, and the lymph nodes were the same as before, and the liver was scattered in the same front.On December 1, 2021, the abdominal CT shows that the peritoneal and lachesis are the same as before.On January 3, 2022, the AFP was reviewed to 74.3 ng/ml.
In the case where the patient received chemotherapy and PFS did not benefit well, the second line adopted a "de-chemotherapy" solution and received Apatinib + PD-1 monoclonal anti-anti-anti-resistance to achieve continuous PR.At present, the second-tier PFS is more than 20 months, and OS has exceeded 24 months. The role of Apatinib and PD-1 monoclonal anti-anti-resistance in HAS is worth further discussion.
The first release of this article: the medical world tumor channel
Author of this article: Dali
Editor in charge: Sweet
- END -
A decoration company uses the "July or Seven Incident" as a poster to attract controversy, lawyer: suspected violation of the advertising law

Polar News reporter MandaIntern Tan DengfangOn July 7, a poster in memory of the S...
Changsha Young Heart Teachers: Hold a special show for dance graduates

Cui Shufang, Director of the Division of the Provincial Department of Education, d...