ASCO releases the 2022 version of advanced lung cancer diagnosis and treatment guidelines!2 major targeting medicines are recommended
Author:Cancer Channel of the Medical Time:2022.07.17
*For medical professionals for reading reference
2022 ASCO Drives Gene -positive IV -stage Lung Cancer Treatment Guide Update
Recently, the Annual Conference of the United States Clinical Oncology Society (ASCO) was held. renew. This article focuses on the field of Phase IV NSCLC therapy for drive gene positive, focusing on two studies, which provides strong evidence support for this guide update, and also proposes new diagnosis and treatment drugs for clinical practice.

Screenshot of the guide
Alk positive NSCLC patients add new choices
01
What are the most effective treatment methods for ALK reunion and previously treated NSCLC patients?
The ASCO Guide's answer to clinical issues 3 (the original guide's clinical question 3) answered a newly added first -line therapeutic drug -Loladinib. The new version of the guidelines clearly stated in the proposal 3.1 that for the ALK genes, PS is 0-2 and previously unprecedented NSCLC patients, clinicians should provide Elitinib or Bukatinib (based on evidence-based evidence; benefits are greater than greater than greater than evidence; benefits are greater than greater than more than evidence; benefits are greater than more than more than evidence; Disadvantages; Evidence Quality: High; Recommended intensity: Strong recommendation) or Loladinib (based on evidence -based evidence; benefits greater than disadvantages; evidence quality: low; recommendation intensity: weak). see picture 1.

figure 1
Lolatinib is the third -generation inhibitor of intercordnic lymphoma kinase (ALK). It was based on clinical I/II results in the clinical clinical in September 2018 and November 2018. [1] was approved in Japan and the United States. Listing, this time in the Guide of the ASCO treatment IV NSCLC, is mainly based on a CROWN phase III clinical random control test. Studies are published in Figure 2.

Figure 2. CROWN Research Published Screenshot
The study is a phase III clinical RCT, which compares the clinical efficacy of Lolatininib and Klitinib. Studies are included in advanced ALK-positive NSCLC adult patients who have not been treated in the past. The PS score is 0-2 and randomly divided into two groups at a ratio of 1: 1. /Sky the treatment; the main ending indicator is the non -progressive survival period (PFS) or the time of death evaluated by the Blind Independent Center, the secondary ending indicator is PFS, Total Survival (OS), and objective relief of the researcher. Objective intracranial relief and safety evaluation results (AE).
In the end, 149 cases were included in the Lolainininini group and 147 cases in the Kizotinininini group. Five patients in the Kizinininini group did not receive treatment and were included in the intention treatment analysis group (ITT). The results of the study show that the median follow -up time of the Loladinini group PFS is 18.3 months, and the Kizininib group is 14.8 months; the proportion of patients who survive and progress without disease in the Loladinib group are in 12 months. 78%(95%CI 70%-84%), while the clipininib group is 39%(95%CI 30%-48%) (HR 0.28; 95%CI 0.19-0.41; P <0.01). See Figure 3A.
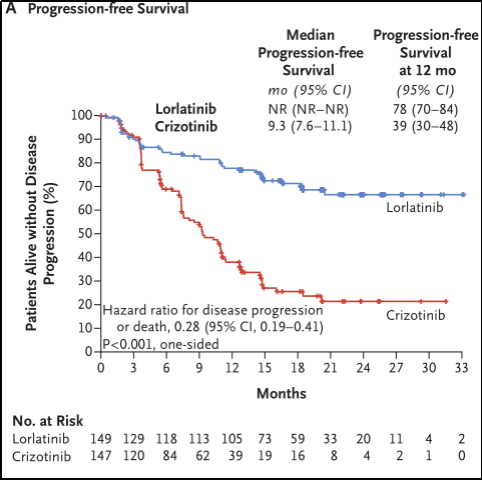
Figure 3A. No progressive survival period
In ITT analysis, Loladinib's central nervous system (CNS) progress time is significantly longer than clizinib. The percentage of surviving patients with no CNS progress in the Lolatinib group was 96%(95%CI 91%-98%), and the Kizininib group was 60%(95%CI 49%-69%) (HR 0.07) ; 95%CI 0.03-0.17). See Figure 3B.
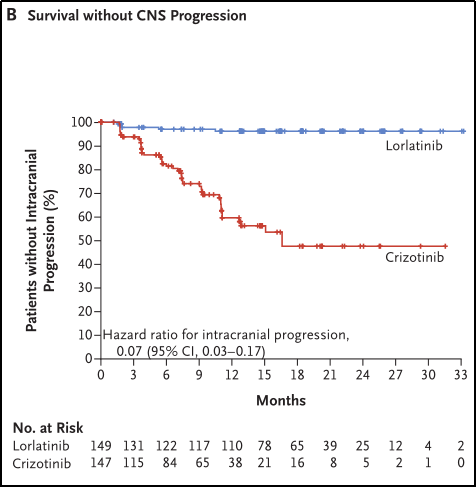
Figure 3B. Survival without CNS progress
In 12 months, the cumulative incidence of CNS progress in the Loladinini group for the first time was 2.8%(95%CI 1.0%-8.1%), while the clipinininib group was 33.2%(95%CI 24.6%-44.7% ) (HR 0.06; 95%CI 0.02-0.18). See Figure 3C.

Figure 3c. The cumulative incidence of CNS progress occurred for the first time
At the end of the data, there were 51 deaths in the ITT crowd (23 cases of the Loladininini group and 28 cases of Kzizininib), and the difference between OS between the group was not significant (HR 0.72; 95%CI 0.41-1.25). See Figure 3D.
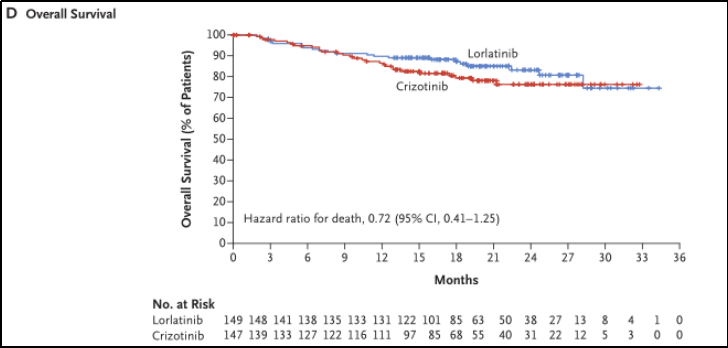
Figure 3D. General survival period
The ratio of 3 or 4 AEs in the Lolatininib and Kizotinini groups was 72%and 56%, respectively. Among them, the most common adverse reactions of the Lolatinib group are elevation of triglyceride levels, increased weight, elevated cholesterol levels, and high blood pressure. The most common in the Kizotinib group is AST and ALT levels. The incidence of severe AE in the two groups was 34%(Lorantinib group) and 27%(clipininib group); a total of 14 patients with fatal adverse incidents, and 7 groups each saw 7 cases.
In summary, the third -generation ALK inhibitor Lolatinib is significantly prolonged compared to the first -generation inhibitors clipininib, but OS has no significant differences (see Figure 3A, 3D).
Although there is evidence that Lolatini is effective in patients with ALK -positive IV NSCLC, and this guidelines are included in the choice of first -line treatment of this type of patient It is cognitive changes and emotional disorders (no matter any level), as well as higher levels of triglyceride, weight gain, increased cholesterol level, and hypertension. RET Row: Prartinib adds new evidence
02
What are the most effective treatment for RET re -exclusive patients?
This time the ASCO Guide updated, the recommendation of the 2021 recommendation for clinical issues 11 (Clinical Question 11 of the original guide) 11.1 and 11.3 reorganization, pointing out "for NSCLC patients who are 0-2 and previously unprecedented NSCLC patients, clinicians who have not been treated in the past. It can provide SelperCatinib or Platinib (evidence type: informal consensus; evidence quality: low; recommendation intensity: medium), and recommend Prartinib and Selpercatinib as side -by -side evidence. See Figure 4.
Figure 4

03
What are the most effective second-line therapy for patients with Phase IV NSCLC with RET Reton PS 0-2?
The recommendations of clinical issues 12 (the original guideline's Clinical Question 12) are reorganized 12.2 and 12.3. It is recommended that "RET re -arranged patients who have not received RET targeted therapy, clinicians can provide SELPERCATINIB (informal consensus; Evidence quality: low; Recommended; recommendation Power: medium) or Platinib (informal consensus; evidence quality: low; recommendation strength: weak) ", merged Platinib and Selpercatinib into the suggestion 12.2. See Figure 5.
Figure 5

Pradinib is a highly efficient oral selective RET inhibitor developed by the Cornerstone Pharmaceutical. As early as 2020, based on ARROW -based research, it was accelerated by FDA for NSCLC and thyroid cancer. [2], at 2022, at 2022 On March 24th, it was also approved by my country's State Drug Administration. The Pradini was mentioned on the "front row" on the clinical issue of ASCO Guidelines 11 and 12, which was also based on this Arrow's queue study. See Figure 6.
Figure 6.Rrow Research Published Screenshot

This study is a multi -queue, open label, and phase 1/2 studies. At present, only the documents have only reported the complete research results of the two -stage test:
Study in patients with age ≥18 years old, suffering from local advanced or metastatic solid tumors and RET fusion positive, PS is 0-2 (the post-scheme correction is 0-1); in the 2nd trial, patients accepted Pula orally Pula orally Pula Treatment of 400mg/day for dietib.
The main ending indicators of the 2nd stage test are objective relief rates (ORR) and safety evaluation results; secondary ending indicators are to alleviate duration (DOR), clinical benefit rate [CR/PR/Disease Stability (SD) continues ≥16 weeks Patient ratio], the proportion of patients with DCR, CR/PR/SD), intracranial tumor relief rate, PFS and ECG.
The results of the study showed that among patients who had previously received platinum chemotherapy (87 cases):
A total of 53 cases of diseases (61%; 95%CI 50%-71%) of 87 patients were relieved, of which 5 were CR; the disease control rate was 91%(95%CI 83%-96%), and clinically obtained it. The interest rate is 69%(95%CI 58%-79%), see Figure 7;
Figure 7

The median time that was relieved for the first time was 1.8 months (IQR 1.7-1.9). From the first relief to the mid-level follow-up time of 12.9 months, the mid-position Dor did not reach (95%CI 15.2-inestimable), see Figure 8;
Figure 8

1. When the median follow-up time is 14.7 months (IQR 12.7-18.4), the mid-bit PFS is 17.1 months (95%CI 8.3-22.1), and 42 patients have developed or died of diseases, as shown in Figure 9;
Figure 9
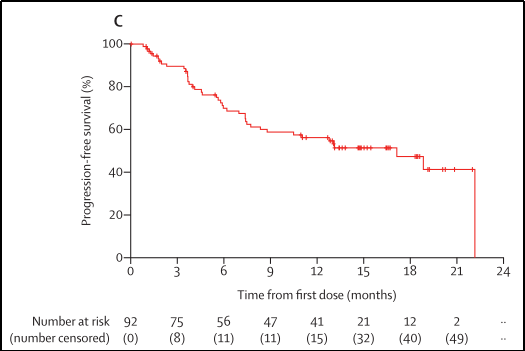
2. When the median follow-up time was 17.1 months, the median OS did not reach (IQR 14.6-20.3), and 25 patients died;
Of the 3.83 patients, 79 patients observed the tumor to shrink from the baseline level.
Among the patients who have not been treated in the past (27 cases):
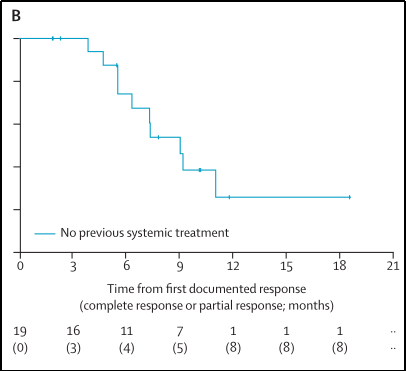
1.27 of the patients who had not been treated in the past relieved (70%; 95%CI 50%-86%), of which 3 were CR; the disease control rate was 85%(95%CI 66%-96%-96% ), The clinical benefit rate is 70%(95%CI 50%-86%), see Figure 7;
2. When the median follow-up time is 10.2 months (IQR 7.8-11.8), the median Dor of all patients to relieve patients is 9.0 months (95%CI 6.3-inestimable), see Figure 10;
Figure 10
3. When the median follow-up time is 11.6 months (IQR 11.0-17.3), the mid-position PFS is 9.1 months (95%CI 6.1-13.0), and 17 patients have developed or died of diseases, as shown in Figure 11;
Figure 11
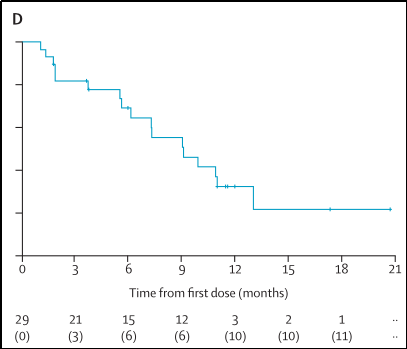
4. When the median follow-up time is 13.6 months (IQR 13.0-17.6), the median OS has not been achieved, and 5 cases of the dead are.
In terms of safety, 216 of the 233 patients appeared AE related to the treatment, the most common is the reduction of neutral granulocytes, hypertension and anemia; 55 cases of severe treatment related AE, the most common are pneumonia and anemia, anemia, anemia. A decrease in neutral granulocytes. See the figure. All in all, ARROW's research shows that no matter whether it has been treated with platinum or not, Platini has good tolerance and lasting clinical activity (including intracranial activity) in the RET fusion -positive NSCLC patients - The ORR of patients with platinum chemotherapy is 61%and the median PFS is 17.1 months. The ORR among patients who have not been treated in the past are 70%and the median PFS is 9.1 months.
Studies have shown that the proportion of genetically -driven genes in patients with NSCLC accounted for 73.9%[3]. In addition to the ALK and RETs mentioned above, there are also EGFR, ROS1, NTRK and MET, etc. Patients with most use targeted therapy have greatly delayed the progress of the disease. However, drug resistance is an inevitable challenge for targeted therapy. Researchers are constantly developing new drugs to try to solve this problem. For example, ALK inhibitors are from the generation of cizotinib to the later Elitinib that appears later. , Bukatinib, developed to the current three generations of Loladinib.
In the future, the treatment of tumors may shift to a solution to multiple therapy combinations (such as the current hot topic immunotherapy combined with targeted therapy) [4]. The update of this guide means that the medical community has gained a new step in the precise treatment of driving gene -positive NSCLC. At present, developing more effective drugs is still crucial to improving the survival rate of driving gene positive patients.
references:
[1] Syed YY.LORLLATINIB: First Global Approval.drugs.2019 Jan; 79 (1): 93-98.doi: 10.1007/S40265-018-1041-0.pmid: 30604291..
[2] Kim J, Bradford D, Larkins E, et al.fda approval summary: PralSetinib for the Treatment of LUNG and Thyroid Cancers With Ret Generations or Fusions.clin Cancer Res. 5456.DOI: 10.1158/1078-0432.CR-21-0967.epub 2021 May 27.pmid: 34045295.
[3] Wen S, Dai L, Wang L, Et Al.Genomic Signature of Driver Genes Identify by Target Next-Generation Sequencing in Chinese Non-Small Cell LUNG CANCEROGIST, 2019,24 (11): E1070 -E11111111111111111111111111111111111111108 -E11111111108E111111110 : 10.1634/theonCologist.2018-0572
[4]Tian X,Gu T,Lee MH,Dong Z.Challenge and countermeasures for EGFR targeted therapy in non-small cell lung cancer.Biochim Biophys Acta Rev Cancer.2022 Jan;1877(1):188645.doi:10.1016/ j.bbcan.2021.188645.epub 2021 nov 15.pmid: 34793897.
The tumor guide you want
Please pay attention to the doctor station 注
1. Scan the QR code below
2. Click to download the app

3. Open the doctor station app and click on the upper right corner

4. Find the tumor in the channel
And follow
Download the doctor's station app and subscribe anytime, anywhere ~
The first release of this article: the medical world tumor channel

Author of this article: I want
Review of this article: Yu Jiangyong Beijing Hospital
Editor in charge: Sweet
- END -
[Suggestion Story of 100 Proposal] Cheng and Qiang: Let the craftsman's spirit take root in the construction industry

Let the artisan spirit take root in the construction industry——Cen Cheng and Qia...
Pass through the sea, and send electricity to the island with a pipe gallery!The latest progress of the project ...
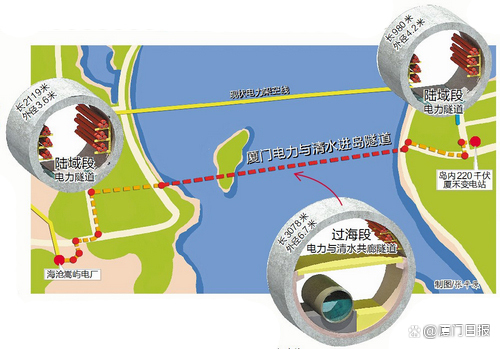
【Electricity】Turn into 2 20 kV cablesIt helps to enter the island's third channe...