The $ 141 billion market is to be developed in the blue ocean, and the departure analysis of the global children's medicine project!
Author:Pharmaceutical economy Time:2022.08.05
At present, in some diseases fields, there is still a lack of dosage forms and products suitable for children. Children's medication (hereinafter referred to as "child medicine") has become the common issue that the medical industry, pharmaceutical government authorities and patients are concerned about in the world. In the United States with a high level of production and supervision, more than 75%of the medicines lack the information of children's medicine, especially in the instructions that lack the amount of use of children's weight or age calculation. Therefore The universal characteristics of children's medication at home and abroad.
For a long time, the value of children's medicine development and market value has been seriously underestimated; according to the paper published in the "Pharmacy and Pharmacology Magazine" in 2017, "Better Medicines for Children: Are We There Yet?" FUNG et al. Published in the "OFF-LABEL Medication Use in Rare Pediatric Diseases in the United States" in the magazine of "Research on". The global childbirth market size is about 97.3 billion US dollars. In 2019, the valuation of the global medicine and vaccine market is about $ 122 billion, and it is expected to reach $ 141 billion in 2025.
This development trend has also promoted the enthusiasm of global pharmaceutical companies to develop children's medicine; on the other hand, governments of various countries have continued to promote the policies of child medicine, the attention of children's medicine safety issues, and the attention of children's special medicines have also greatly promoted children. The development of medicine.
The United States is the earliest country of childhood medicine legislative supervision, and the European Union has been working hard to strengthen children's medicine management and policy support. This article briefly interprets and sort out the relevant regulations of children's medicines in Europe and the United States and the current distribution of global medicine projects.

European and American policy inventory
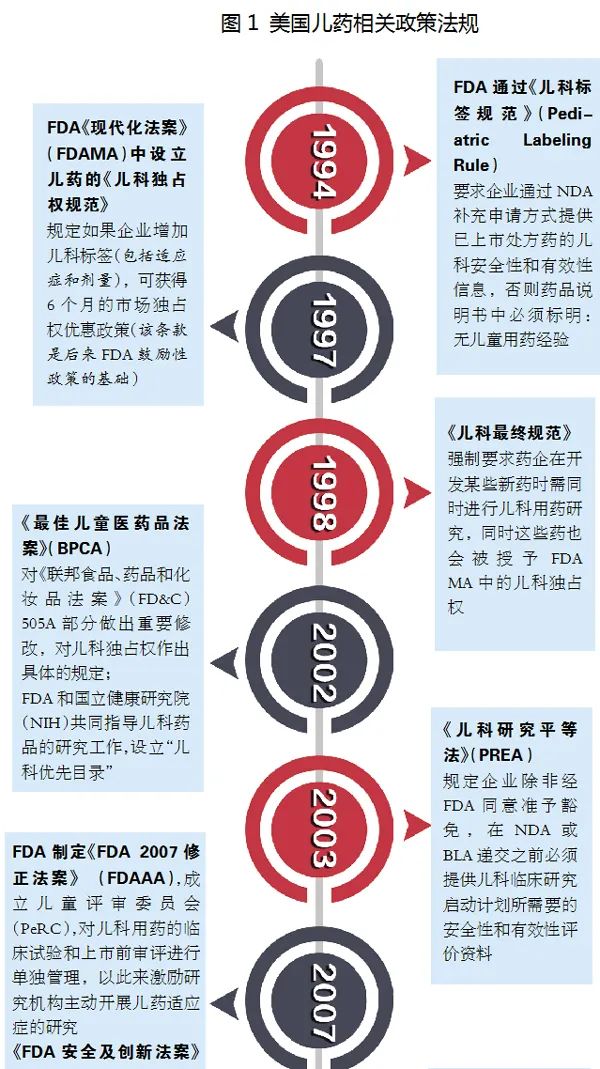
The United States is currently the world's largest children's pharmaceutical market and one of the most sound countries in the pharmaceutical industry. In 1979, the FDA began to require drug labels to provide children's use information. With a complete set of legislative legislation, the research of child medicine accumulated relatively mature policy and regulations.
Since the implementation of the “Pediatric Ewen Rights of Pediatrics” in 1997, according to the data disclosed by the official website of the FDA, as of October 2020, a total of 263 child medicine exclusive rights were granted. Among them, 15 child medicine exclusive rights were awarded in 2019, and 6 exclusive rights of pediatric drugs were awarded in 2020. And the companies that belong to are basically large pharmaceutical companies, small companies or newly founded companies are insufficient in R & D expenses. Generally, it is difficult to control the challenges and dilemma faced by children's medicine development, which also fully reflects the difficulty of child medicine development.
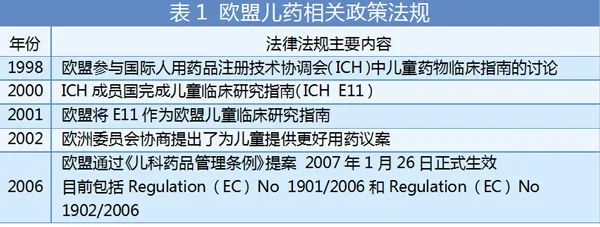
The EU is another huge market for children's medicines. The size and number of their pharmaceutical companies cannot be underestimated. In terms of strengthening children's medicine management and policy support, the European Union has been working hard. In 1997, the European Commission was in an expert round table meeting organized by the EMA organization. After discussing the issue of child medicine, the meeting believed that it was necessary to strengthen legislation and introduce the incentive mechanism of children's drug development.
Here we focus on analyzing the Paediatric Regulation Regulations. The regulations require that starting from July 26, 2008, new drugs launched by European pharmaceutical companies need to pass the clinical trial of children. The clinical trials of the product are described to provide data.
At the same time, EMA specially established a Pediatric Committee (PDCO), which is mainly responsible for evaluating PIP, and is responsible for maintaining the network of clinical research in European children. Since January 26, 2009, new indications, new dosage forms, and new prescription supplementary applications must also include PIP. According to PIP medicines that obtain child medicine data, they can obtain a 6 -month market protection period. Among them, for drugs that treat rare diseases, the protection period can be extended for 2 years.
On the other hand, according to Article 26 of the Pediatric Drug Management Regulations, EMA provides free scientific recommendations (SA) or agreement assistance (PA) for any issues related to the R & D of drugs. These suggestions are generally made by the scientific proposal work group (SAWP) and adopted by Human Pharmaceutical Committee (CHMP). For the request for the development of pediatric medications, members of the Pediatric Committee (PDCO) usually provide scientific suggestions as an expert through the SA program. This regulation has been implemented since 2009.
From EMA and the European Commission's action plan for pediatric drugs, we can see its clear attitude and action plan: determine the needs of pediatric drugs, strengthen the cooperation of decision makers, ensure timely PIP in time The transparency of pediatric drugs.
It can be seen that the EU has established a relatively complete system from legislative, technical guidelines, management procedures, and implementation paths for pediatric drugs.
Distribution in projects
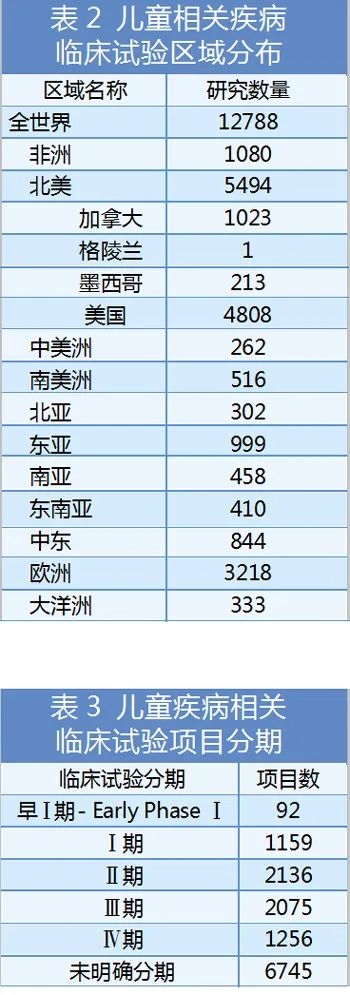
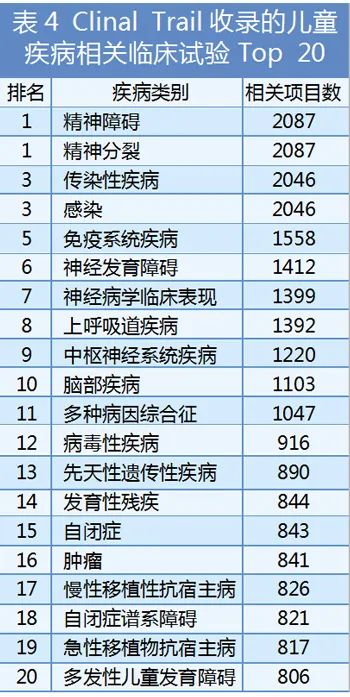
Although the level of policies and regulations related to children's medicine supervision are incomplete, the level of implementation and implementation of childcare supervision is not uniform, and in general, it has continued to improve and improve, and encourage and support the development of child medicine. In such a major environment, the development momentum of children's medicines is becoming increasingly fastest, and the types are more extensive. According to the clinical trials website data, there are nearly 13,000 children's disease -related projects that have declared clinical trials (from not recruited volunteers to phase IV clinical trials). There are more than 2,500 causes of diseases and health effects involved in the project, of which the top 20 disease types are distributed in Table 4.
These data reflect the current status of children's drugs from many aspects and angles. This booming trend is not only a manifestation of the increasingly improved relevant policies and regulations, but also a motivation for urging the continuous improvement of existing policies and regulations.
Whether abroad or in China, in order to seek the standardization of the development and use of children's medicines, combine their own characteristics and advantages, and then learn from each other and learn from each other. It is the right way to cooperate with each other if necessary.
Related &&
Compared with the United States and the European Union, Japan has not established laws and regulations to force children's medicine development. To this end, some scholars use information about the lagging of children's indications between Japan and the European Union in 2021 to investigate the status and characteristics of Japanese children's drug development.
Through the study of drugs approved by Japan from January 2007 to December 2018, it was found that in Japan and the European Union, the number of 105 pediatric indications drugs lags 1017 days (Japan lags). The lag of Class A Class B (medication) and Class L (anti -tumor and immunotherapy) has been significantly improved after 2011; the median is less than half a year. Compared with the drug developed by multiple regional clinical trials in the world, the lag time is significantly shortened compared with the regional development of drugs.
In addition, based on European and Japan's drug information approved by pediatric indications, some scholars evaluated the latest status and characteristics of Japanese child medicine approval in 2020. Their research was included in the drug approved for pediatric indications from 2007 to 2015. The ATC classification method is used to calculate the proportion of children's adaptive drugs and analyze the current status of children's dosage development. Determine the time from adults to pediatric indications. A total of 135 drugs in Europe were approved for pediatric indications, and Japan was approved by 208 species.
In the ATC classification of N (medication system) and J (anti -infective drugs), and with the development of pediatric dosage type, the proportion of drugs approved by children in Japan is lower than that of children's indications in Europe. Essence Except for the drug approved by adults and children's indications at the same time, the most common observation cycle approved by adult adult indications approved to children's indications is more than 12 years, and Europe is 3 to 6 years. These research results show that Japan is indeed promoting the development of children's medication, but the time for Japan from adult adaptation to pediatric indications is longer than Europe. The development of pediatric drugs for certain diseases has a slowly improvement of room for further improvement.
Editor -in -chief: Chen Lina
The "replacement of price" is unstoppable! How does the big variety maintain an advantage?

The semi -annual report of biopharmaceutical companies has been released one after another. It is not as good as "selling water" as a strong entry of CDMO!
Osdowe Wei won the seventh batch of the seventh bid for 0.99 yuan/piece, and the "one product is independent" for pressure! Will Dong Sunshine Medicine be the next Xinlitai?
in
www.yyjjb.com.cn

Insight industry trends
Long press and follow the Medical Economic News
"Medical Economic News" academic public account

Focus on the frontier of tumor academic
Long press and follow the immune time
"Medical Economic News" terminal public account

Record the large event of the pharmaceutical terminal production and menstruation
Long press and follow the 21st Century Pharmacy Newspaper
- END -
How does glue cell affect your emotions

Via: fool mixed plant CDD20The following is the audio of the full text of Miss Sis...
The young guy's thigh was tied to the nail, and the doctor jointly operated the "thigh to fish needle"
The nail gun is a tool commonly used by decoration workers, but if you accidentall...