ALK+NSCLC: It is only one step from "living longer" to "more live"
Author:Cancer Channel of the Medical Time:2022.08.15
*For medical professionals for reading reference
Pogkinib officially entered China's clinical application, helping to comprehensively benefit clinical patients
Tumor targeted therapy has changed the clinical practice of lung cancer, making interior transgender lymphoma kinase (ALK) positive non -small cell lung cancer (NSCLC) has moved towards "precision" and "long survival".
With the continuous approval of new drugs, ALK Centerotinase Inhibitors (TKI) ushered in hundreds of argument. The clinician's choice of first -line treatment of drugs has also been transformed from the efficacy of the drug to the efficacy of the drug, the safety of the drug, the characteristics of the patient's disease (such as whether it is accompanied by brain metastasis), and the drug accessibility.
As a rising star in the ALK TKI world, Bugininib was approved in China in March 2022, and has officially entered clinical application. In terms of the efficacy of the overall population, intracranial relief, and the quality of life of patients, it has greatly improved, bringing a new preferred plan for Alk's positive NSCLC first -line treatment.
The overall crowd realizes "more live" -MPFS is as high as 30.8 months; the PFS rate of 1 year is 90%
Alta-1L research
The head opposite compares the final result of the ALTA-1L study with the efficacy and security of the Bigtinib and Kizininib [1]. The Bugininib group is 24 months, while the corresponding Kizininib group MPFS is only 11.1 months (risk ratio [hr] = 0.48, P <0.0001). The MPFS of the Researcher Evaluation (INV) The MPFS in the overall crowd is 30.8 months, and the Kzizinib group is 9.2 months (HR = 0.43, P <0.0001), and Bogkinib reduced by 57% by 57% The risk of disease progress or death (Figure 1). The objective relief rate (ORR) evaluated by the second point of BIRC (ORR) is 74%(95 ci: 66%-82%), and the median continuous relief time for patients who received the relief after receiving Bogininib for treatment is 33.2 months (95Ci : 22.1-not reached (NR)).
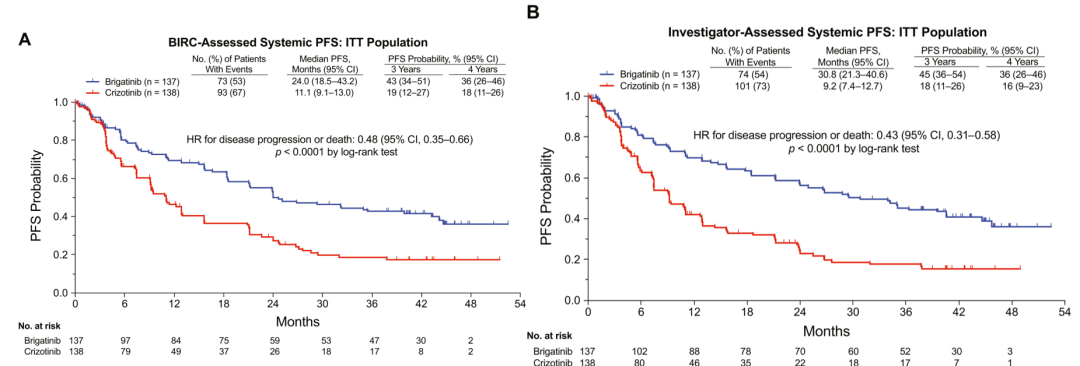
Figure 1.ALTA-L1: The non-progressive survival period evaluated by Bigtinib IRC and researchers [1]
In terms of overall survival period (OS), the 3 -year survival rate of patients in the Buginini group is 71%, and the 4 -year survival rate is 66%. Essence 47%of patients in the Kizotininib group used Bogntinib, and in the sensitivity analysis (MSM method) after cross therapy of the Kidinib group, the risk ratio (HR) of OS was 0.54 (HR) P = 0.023), the risk of death was reduced by 46%(Figure 2).
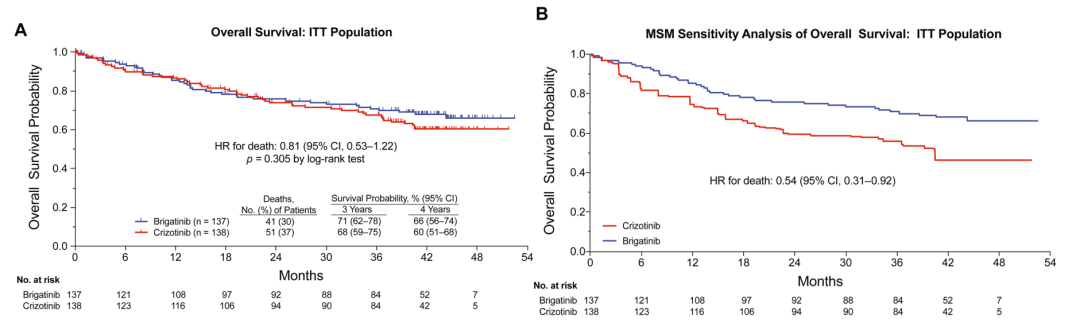
Figure 2.ALTA-L1: Analysis of the total survival period [1]
In addition, in 2022, the American Clinical Oncology Society (ASCO) conference disclosed the analysis of Alta-1L exploration-about the correlation of the degree of relief of target lesions and the ending of survival. Ni has a more significant deep relief (the target lesions are narrowed> 75%), and the proportion of patients in the depth relief is 56%and 34%(p = 0.0005), respectively. In addition, the degree of relief of target lesions can affect the end of the patient's survival. The PFS rate of patients in the in -depth relief group is as high as 56%, and the 3 -year OS rate is as high as 85%(Figure 3). Studies suggest that Pogeni has a better in -depth relief, which is conducive to bringing higher survival benefits to patients.
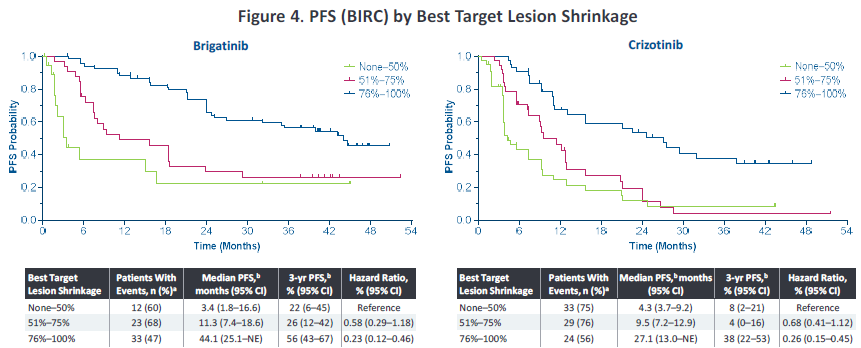
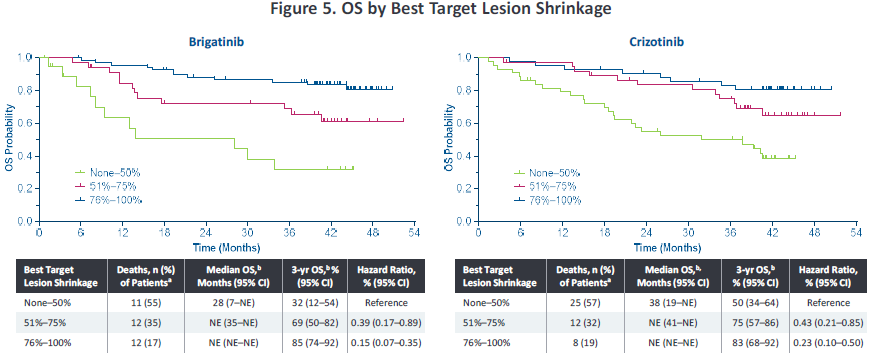
Figure 3. Analyze the total survival period and no progressive survival period based on the maximum reduction ratio of target lesions [2]
J-Alta Research
In addition to seeing the long-lasting benefits brought by Boginib in Alta-1L, J-Alta studied the success of Alta-1L in the Japanese population. In 2022, the ASCO meeting announced the final data of J-Alta [3], which first disclosed the result of the TKI preliminary governance queue of Asia (Japan). The results showed that the 1-year PFS rate of the 1-year PFS ratio of Boginib in the ALK TKI initial governance queue was 90%(90%CI: 75%-96%), and the 2-year PFS rate was 73%(90%CI: 55%55% -85%), the mid -bit PFS did not reach (Figure 4). The 1-year survival rate reaches 97%(95%CI: 80%-100%), and the median total survival period (OS) has not yet reached (95%CI: NR). In addition, the ORR evaluated by IRC is as high as 97%(95%CI: 84%to 100%). The study provided more sufficient evidence for the Asian population to use Boginib, which further consolidated the advantages of Pogkini in front -line treatment.
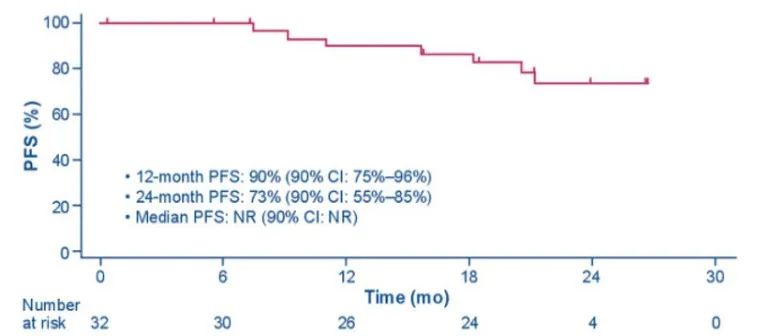
Figure 4.j-alta: The non-progressive survival period of Alk TKI's initial governance queue [3]
Patients with brain metastases can also be "lived longer" -the 4 -year OS rate is as high as 71%
Brain metastases have always been an important constraints for targeted drugs. Infection, intracranial metastases greatly affects the survival time of patients, and has become the bottleneck of ALK -positive NSCLC to further increase the survival rate. Control brain metastases is also an important mission of ALK inhibitor.
Alta-1L research
Alta-1L studies have confirmed that the use of Bogininib can make patients with brain metastases with long-term survival. The results of the alta-1L study showed that the MPFS of patients with baseline brain metastasis reached 24.0 months (VS Cizidinib 5.6 months), reducing 75%of the risk of disease progress or death (Figure 5). Bigetinib's intracranial ORR reaches 78%(26%of VS Cizidinib). For patients with intracranial lesions, it has a continuous relief time of 27.9 months, and the control group is only 9.2 months. The HR of the OS for the patients with brain metastases is 0.43 (P = 0.020), which indicates that the drug has benefited from the survival of patients with brain transfer. It is still as high as 71%(44%of VS Cizyininib). A is based on the researcher's assessment of the baseline.
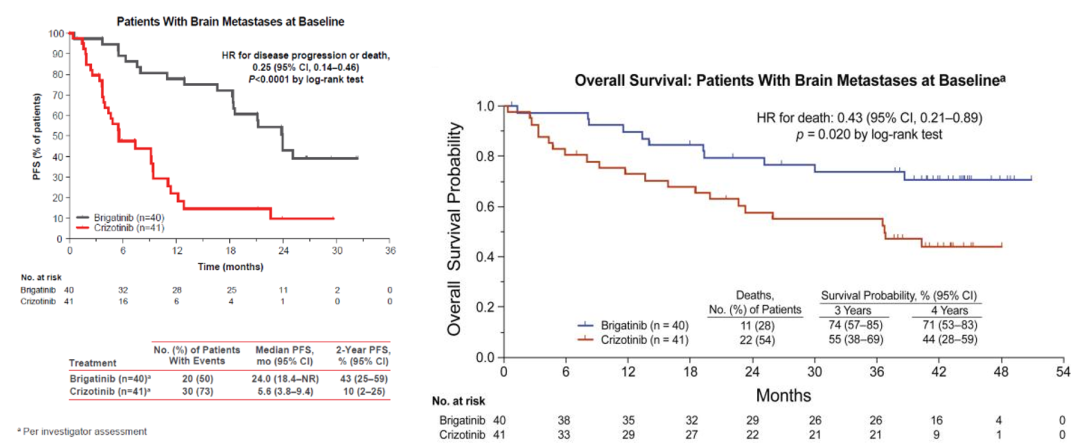
Figure 5.ALTA-L1: The non-progressive survival and overall survival period of patients with baseline brain metastases [1]
J-Alta Research
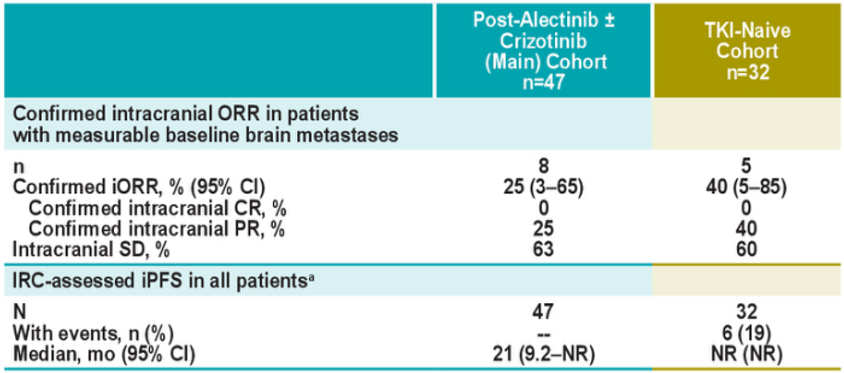
The J-ALTA study once again verified that Boginib is effective for ALK-positive NSCLC brain metastases. Among the five patients with measuring brain transfer patients, the intracranial ORR that confirmed by IRC was 40%(95%CI: 5%-85%) [3].
Figure 6.j-alta: intracranial relief in patients with brain metastases [3]
Provide patients with a "better living" choice -the first ALK TKI to achieve a significant benefit of the quality of life
ALK -positive late -stage NSCLC patients are relatively young and prone to brain metastases. Patients have changed from the original pursuit of "living longer" to "better live." Therefore, the improvement of drug safety and health -related life (HRQOL) is becoming more important.
Alta-1L research
In the alta-1L study [1], the median time of the worsening of the patients in the Bugininib the treatment group was 26.7 months, and the median time of the deterioration of the health state/quality of life (GHS/QOL) was 26.7 months, and the Kazininib was 8.3 months. (HR = 0.69, P = 0.047), it is prompted that Pogkinib can significantly improve the overall HRQOL and delay the time of the decline in life. Compared with Cizitinib, the Pogkinib significantly delays the deterioration time of multiple dimensions. Such as fatigue, GI -related symptoms (nausea and vomiting, reduced appetite, constipation), and emotional and social functions (P <0.05).
In terms of safety, the most common adverse reactions of Bogininib in Alta-1L research are gastrointestinal reactions, elevated hemoglip phosphate (CPK), cough and elevated transaminase (Figure 7). The security data of the front -line treatment is consistent with other studies reported in the past. No new adverse events have been seen, and the tolerance is good.
EORTC QLQ-C30: European Cancer Research and Treatment of Quality Survey of Life-Core 30
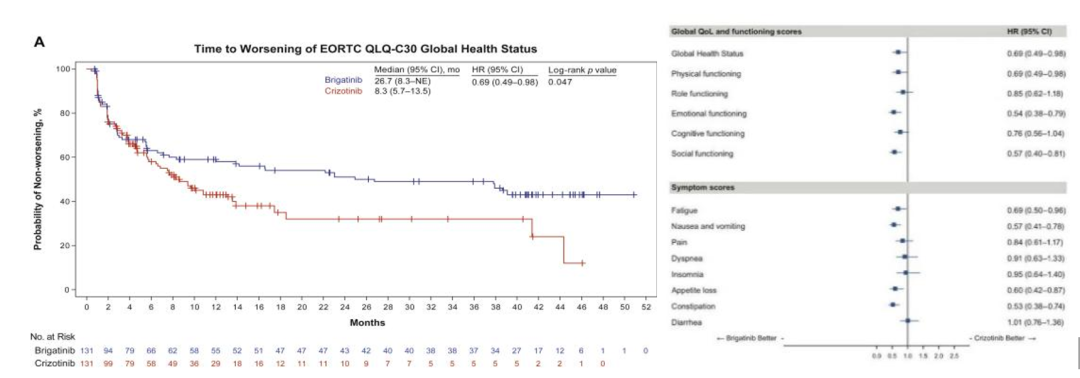
Figure 7.ALTA-1L: EORTC QLQ-C30 score [1]
J-Alta Research
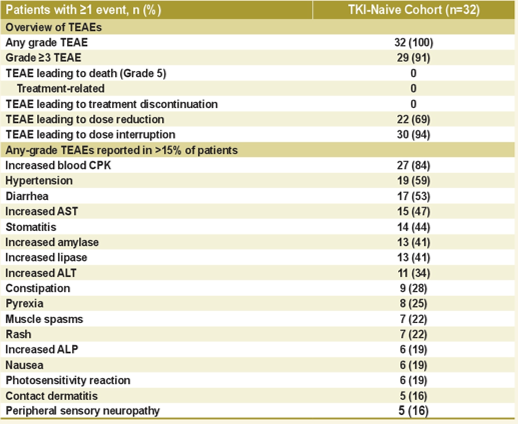
J-ALTA research crowd uses the same tolerance to the first line of Poginib, and no patient has a treatment related to the treatment of drugs that need to be discontinued (Figure 8). Four patients (12.5%) patients reported intermediate pneumonia (ILD)/pneumonia, and most ILD/pneumonia cases were improved after stopping the use of Poginib or not steroid treatment [3].
Figure 8.J-Alta: Security Final Analysis
summary
ALK -positive NSCLC has approached "slow disease" management, and long -term survival of patients is no longer a luxury. In the choice of clinical treatment, doctors need to comprehensively consider many factors: efficacy, adverse reactions, and patient's economic tolerance, so as to formulate a personalized first -line treatment plan for patients. At present, the first -line medication of ALK -positive non -small cell lung cancer recommended by NCCN guidelines [4] includes: Cizibinib, Sereidib, Alantinib, and Loladinib. Boginib.
In response to ALK -positive NSCLC patients have the characteristics of relatively younger diseases and prone to brain metastases. As a member of ALK TKI, in addition to bringing longer relief and higher survival time, it has also realized it. The quality of long survival and quality of life has been improved. As Boginib officially entered the clinical application in China, it is expected that it can quickly enter medical insurance, improve the availability of drugs, and benefit more patients!
references:
[1] Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in Anaplastic Lymphoma Kinase (ALK) Inhibitor-Naive Advanced ALK-Positive Non-Small Cell Lung Cancer: Final Results of the Phase 3 ALTA-1L Trial. J Thorac oncol. 2021; 16 (12): 2091-2108.
[2] D. Ross Camidge, Hye Ryun Kim, Myung-Ju Ahn, et al. Association of depth of target lesion response to brigatinib with outcomes in patients with ALK inhibitor-naive ALK+ NSCLC in ALTA-1L. Journal of Clinical Oncology 2022 40:16_suppl, 9072-9072[3] Pingkuan Zhang, Toru Kumagai, Tatsuya Yoshida, et al. Brigatinib in Japanese patients (pts) with ALK+ NSCLC: Final results from the phase 2 J-ALTA trial. J Clin Oncol 40, 2022 (SUPPL 16; ABSTR 9075)/2022 ASCO POSTER BD #62
[4] NCCN ClinicalPractice Guidelines in onCology-Non-Small Cell LUNG CANCER, Version 1. [J].
Approval number: VV-MEDMAT-72086
Approved date: August 2022
statement:
This information aims to help medical professionals better understand the latest progress in the field of related diseases. The content of the information released on this platform does not mean that it agrees with its description and view, only to provide more information. If the copyright issue is involved, please contact us, we will deal with it as soon as possible.
The information provided by this information cannot replace professional medical guidance in any way, nor should they be considered diagnosis and treatment suggestions. If such information is used to understand the purpose other than information, this platform, authors and Takeda do not assume relevant responsibilities.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
[2022 ASCO] Revolutionary new │MHSPC field wonderful highlight express delivery

*For medical professionals for reading referenceOn June 3, 2022, the annual confer...
Lianhua Qing Plague good prevention and control advantage

Recently, the epidemic in Hainan and other places in my country has reminded every...